
The introduction and development of corneal cross-linking (CXL), a technique that stiffens corneal tissue through applications of riboflavin and ultraviolet A irradiation, has been a boon to ophthalmology over the past decade-plus.1 Many European surgeons have been treating patients with CXL throughout this period, but for US ophthalmologists, the procedure became available only recently outside of clinical trials, with the FDA’s approval of the KXL System (Avedro) this past year.
AT A GLANCE
- At present, there is no technology available to identify keratoconus patients until they have already experienced change and lost some quality of vision; likewise, those who are at risk of further change cannot clearly be distinguished from those who are not at risk.
- Recently, interest has turned to finding ways to directly measure corneal strength, both to detect weakness and to measure the strengthening effect of CXL. The most promising research today is looking at devices or technologies that can measure specific location-based corneal properties.
- Perhaps the most promising technology for noninvasive measurement of the mechanical strength of the cornea, Brillouin microscopy has the ability to measure the intrinsic strength or stiffness of the cornea without air puffs or other perturbations and to see the relative stiffness of the cornea at different layers and locations.
The goal of CXL is to prevent or halt the progression of corneal ectasia, whether caused by a disease such as keratoconus or by an iatrogenic source such as corneal refractive surgery. Although the incidence of corneal ectasia is low, it is one of the most problematic complications of corneal refractive surgery, and identifying risk for ectasia preoperatively is perhaps the most important goal in screening candidates for these surgeries.2
THE PROBLEM
The problem with current methods for detecting ectasia risk is that there is a gap between the tools we have and what we would like to have. We lack a direct measurement of focal corneal strength and stiffness. We would like to be able to identify patients with weak corneas, with a predisposition for the shape change that occurs in iatrogenic ectasia or keratoconus. If we could detect corneal weakness in patients with subclinical keratoconus, we could treat them using CXL before they experience significant vision loss. Similarly, if we could detect it with certainty and without significant false positives or negatives in refractive surgery candidates, we could avoid surgery in those patients or, perhaps, strengthen their corneas preoperatively with CXL to allow them to enjoy the benefits of surgical refractive correction safely.
Unfortunately, we do not have reliable methods to measure corneal stiffness or strength clinically at present. We rely instead on morphologic characteristics such as abnormal curvature, corneal thinning, or epithelial thinning. These methods can diagnose existing ectatic disease, but they cannot help us much to predict future change. In other words, we cannot identify patients until they have already experienced change and therefore already have some loss in the quality of their vision. Even in these patients, we cannot clearly distinguish those who are at risk of further change from those who are not. For this reason, development of new diagnostic modalities for corneal ectasia is the goal of numerous current research efforts.
THE SEARCH
Risk factors for ectasia after corneal refractive surgery include signs of forme fruste keratoconus on corneal topography, elevated posterior float values on corneal tomography, high myopia, preoperative corneal thickness less than 500 µm, and residual stromal bed thickness less than 250 µm. Even with attention to these risk factors, however, ectasia can still occur after LASIK.3
Almost a decade ago, my colleagues and I described and validated an ectasia risk scoring system.4,5 We found that this system, which assigns points in a weighted fashion to several variables—topographic pattern, predicted residual stromal bed thickness, age, preoperative corneal thickness, and manifest refraction spherical equivalent—was an effective method of identifying eyes at risk for post-LASIK ectasia that offered significant improvement over earlier screening strategies.4,5 Still, an objective measure of corneal weakness would be invaluable in better predicting a patient’s risk for ectasia. Currently, our screening algorithms err on the conservative side to avoid ectasia risk, but this certainly prevents many patients from receiving the benefits that refractive surgery offers.
The change in the Kmax value is one of the indicators that many clinicians have used when evaluating the efficacy of cross-linking (Figure). This is not always reliable, however, as there can be significant fluctuation in this single indicator in abnormal corneas. Now, we have begun looking at other topographic indicators such as the overall steepest area of the cornea—not just a single point, as with Kmax, but regional steepness. Other indicators include the amount of slope change from steepest to flattest areas and the overall regularity of the corneal surface.
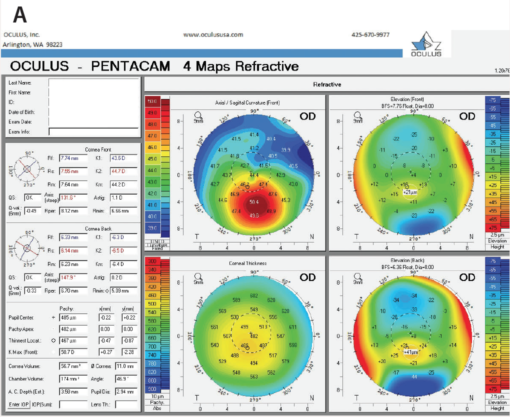
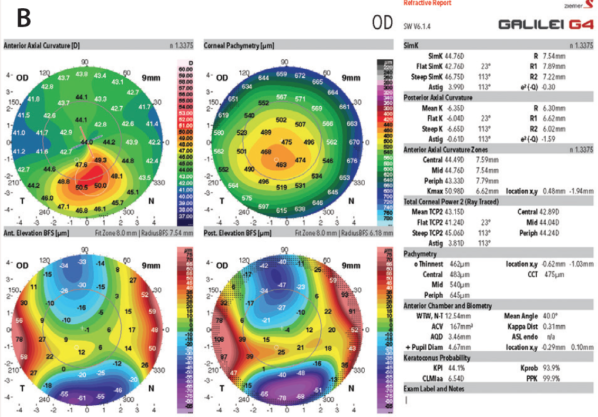
Figure. The two most common imaging techniques currently used to identify ectasia risk are the Pentacam (A; Oculus Surgical) and the Galilei (B; Ziemer Ophthalmic Systems). Both display maximum keratometry (Kmax) directly.
THE SOLUTIONS
Recently, interest has turned to finding ways to directly measure corneal strength, both to detect weakness and to measure the strengthening effect of CXL.
Air-puff measurements. Two air-puff–based technologies originally developed to measure corneal hysteresis or corneal resistance factor in glaucoma have been evaluated for use in the context of CXL. In work led by William J. Dupps Jr, MD, PhD, at the Cleveland Clinic, we evaluated some investigator-derived custom variables for the Ocular Response Analzyer (Reichert Technologies) that show an increase in corneal stiffening after CXL. However, the changes observed in corneal deformation response were small and seemed to underestimate the biomechanical changes induced by CXL.6
Recently, Vinciguerra and colleagues evaluated a biomechanical index generated by a similar device, the Corvis ST (Oculus Surgical), to identify keratoconic patients. In a multicenter study including more than 650 patients, the investigators found that the Corvis biomechanical index, based on corneal thickness profile and deformation parameters, was highly sensitive and specific in distinguishing healthy from keratoconic eyes.7 Initial reports suggest, however, that it is still challenging to use the Corvis biomechanical index to distinguish eyes that have and have not undergone CXL and to distinguish early to mild keratoconus from normal eyes.
Optical coherence elastography. Another technology based on dynamic measurement is optical coherence elastography, a technique using optical coherence tomography imaging that is being investigated by Ford et al.8 In this nondestructive approach, the displacement of optical features within the cornea is tracked in two dimensions in a dynamic fashion, yielding information that may be relevant in the early diagnosis of corneal ectatic disease. This technology is still in the research setting at present.
Brillouin microscopy. One of the most promising technologies for noninvasive measurement of the mechanical strength of the cornea is Brillouin microscopy. This exciting research tool is in use in prototypes and not yet commercially available.
The problem for clinicians is that the diagnostic devices we have available measure global properties of the cornea, but significant research indicates that corneal ectasia is a focal process. If we measure the overall corneal properties, therefore, we are going to miss early focal changes. This is why the most promising research today is looking at devices or technologies that can measure specific location-based corneal properties.
Whose Bright Idea Was That?
By Laura Straub, Editor-in-Chief
The French physicist Léon Nicolas Brillouin is credited with discovering Brillouin zones, a central concept in the physics of solids.
Born in 1889, Brillouin first studied physics at the École Normale Supérieure in Paris, then at the Institute of Theoretical Physics in Munich, Germany, and then at the University of Paris. Presenting his doctoral thesis on the quantum theory of solids to a panel that included Marie Curie, Brillouin proposed an equation of state based on the atomic vibrations that propogate through it.1 Also in his thesis, Brillouin defended how monochromatic light waves propagate through a solid and interact with acoustic waves. Here, he showed that the scattered wave is composed of the sum of three components—one at the frequency of the incident wave and two at frequencies located relative to it—and that their separation is dependent on the scattering angle.
Brillioun scattering, the interaction between an electromagnetic wave and the phonon, polaron, and magnon crystalline lattice waves, can be used to measure the energies, wavelengths, and frequencies of quasiparticles.
1. http://www-llb.cea.fr/presllb/leonbrillouin_e.pdf. Accessed June 27, 2017.
Brillouin microscopy has been used in vivo in humans, but because the measurement process is fairly slow, it is not yet clinically viable. The technology has the ability, however, to measure the intrinsic strength or stiffness of the cornea without air puffs or other perturbations. Measuring keratoconus patients with this technology before and after CXL could potentially yield a tremendous amount of information. Brillouin microscopy also provides 3-D resolution, so it allows us to see the relative stiffness of the cornea at different layers and locations.
Giuliano Scarcelli, PhD, and Seok Hyun Yun, PhD, did the foundational work with this technology in ophthalmology with colleagues at Massachusetts Eye and Ear Infirmary.9,10 Working with donor corneas, these researchers detected notable differences between healthy and keratoconic corneal tissues using Brillouin measurements. Their work showed that mechanical loss in the keratoconic corneas was primarily concentrated within the area of the cone and that, outside the cone, the Brillouin shift was comparable with that in normal corneas.9 They subsequently used the same technology to map the elastic modulus of normal and keratoconus patients in vivo and found distinctive biomechanical features that differentiate normal from keratoconic corneas.10
Now at the University of Maryland, Dr. Scarcelli continues to pursue the promise of this technology, not only in ophthalmology but also in other fields of medicine. Based on the promising work he and his colleagues have done, we are working toward performing research using Brillouin microscopy in collaboration with Dr. Scarcelli at the University of Southern California.
As noted, in using Brillouin microscopy to determine the relative strength of the cornea at a variety of locations, Scarcelli et al were able to demonstrate that the cone region in keratoconus was abnormal but the surrounding regions were similar to those of normal patients.9 This gives us hope that this Brillouin technology might be able to identify focal changes in the cornea before they become manifest with our other testing.
THE FUTURE
Brillouin microscopy has been used only in prototypes to date, so it is some distance from practical clinical use. Still, it holds great promise that has a significant chance of reaching the clinic in the foreseeable future.
We know what CXL can do based on destructive laboratory testing. If questions remain regarding what CXL can do in patients, this is not because of any fault in the procedure itself, but rather because we do not have the devices we need to measure its effects in living human corneas, as opposed to destructive laboratory tests. This is the gap that researchers are trying to close with new and promising diagnostic technologies such as Brillouin microscopy and optical coherence elastography.
1. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620-627.
2. Dupps WJ Jr. Ectasia risk: a multifactorial conundrum. J Cataract Refract Surg. 2015;41(4):699-700.
3. Klein SR, Epstein RJ, Randleman JB, Stulting RD. Corneal ectasia following laser in situ keratomileusis in patients without apparent preoperative risk factors. Cornea. 2006;25:388-403.
4. Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115:37-50.
5. Randleman JB, Tratler WB, Stulting RD. Validation of the ectasia risk score system for preoperative laser in situ keratomileusis screening. Am J Ophthalmol. 2008;5:813-818.
6. Hallahan KM, Rocha K, Roy AS, et al. Effects of corneal cross-linking on ocular response analyzer waveform-derived variables in keratoconus and postrefractive surgery ectasia. Eye Contact Lens. 2014;40(6):339-344.
7. Vinciguerra R, Ambrósio R Jr, Elsheikh A, et al. Detection of keratoconus with a new biomechanical index. J Refract Surg. 2016;32(12):803-810.
8. Ford MR, Dupps WJ Jr, Rollins AM, et al. Method for optical coherence elastography of the cornea. J Biomed Opt. 2011;16(1):016005.
9. Scarcelli G, Besner S, Pineda R, Yun SH. Biomechanical characterization of keratoconus corneas ex vivo with Brillouin microscopy. Invest Ophthalmol Vis Sci. 2014;55(7):4490-4495.
10. Scarcelli G, Besner D, Pineda R, Kalout P, Yun SH. In vivo biomechanical mapping of normal and keratoconic corneas. JAMA Ophthalmol. 2015;133(4):480-482.




