Fueled by an influx of investment capital in recent years and a streamlined regulatory review process, the number of ophthalmic products approved or introduced in 2019 continued to rise steadily throughout the year. According to recent figures from pharmaceutical market research company Strategic Transactions, venture capital investment in ophthalmology exceeded $1.5 billion in 2019, a new record.1 The number of deals completed in ophthalmic pharmaceutical and device companies also reached record levels.
The major industry announcements and advancements in ophthalmology in 2019 included the first approval of a trifocal IOL in the United States; new surgical methods to treat postoperative pain and inflammation; and major upgrades to refractive lasers, OCT, and devices designed to streamline a surgeon’s refractive cataract surgery workflow.
Below is a list of some of the ophthalmic drugs, devices, and IOLs that were approved, introduced, or commercially launched from January through press time for this issue.
OPHTHALMIC DRUGS
October 2019
Beovu (Novartis)
Novartis received FDA approval for Beovu (brolucizumab-dbll) injection for the treatment of wet age-related macular degeneration (AMD). Beovu is the first anti-VEGF agent approved by the FDA to offer both greater fluid resolution versus aflibercept and the ability to maintain eligible wet AMD patients on a 3-month dosing interval immediately after a 3-month loading phase, according to Novartis.

Cequa (Sun Pharmaceutical)
Sun Pharmaceutical commercially launched dry eye drug Cequa (cyclosporine ophthalmic solution 0.09%) in the United States. Cequa, which offers the highest concentration of cyclosporine for ophthalmic use approved by the FDA, is the first and only FDA-approved cyclosporine treatment delivered with nanomicellar technology, which the company says helps to improve the bioavailability and physicochemical stability of cyclosporine.
July 2019

Dextenza (Ocular Therapeutix)
Ocular Therapeutix announced the US commercial launch of Dextenza (dexamethasone ophthalmic insert 0.4 mg) for the treatment of ocular inflammation and pain following ophthalmic surgery. Dextenza is the first FDA-approved intracanalicular insert that enables the delivery of a drug to the surface of the eye, obviating the need for a regimen of steroid eye drops.
June 2019
Soliris (Alexion Pharmaceuticals)
The FDA approved Soliris (eculizumab) injection for intravenous use for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adults who are antiaquaporin–4 (AQP4) antibody positive. Soliris provides the first FDA-approved treatment for NMOSD, an autoimmune disease of the central nervous system that mainly affects the optic nerves and spinal cord.
April 2019
Generic Loteprednol Etabonate (Akorn)
Akorn received an abbreviated FDA new drug application (NDA) approval for loteprednol etabonate ophthalmic suspension 0.5%. Loteprednol etabonate 0.5% is indicated for the treatment of postoperative inflammation following ocular surgery and the treatment of steroid-responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe.
March 2019

Rocklatan (Aerie Pharmaceuticals)
Aerie Pharmaceuticals received FDA approval for Rocklatan (netarsudil and latanoprost ophthalmic solution 0.02%/0.005%) to reduce elevated IOP in patients with open-angle glaucoma or ocular hypertension. Rocklatan is a once-daily eye drop that is a fixed-dose combination of latanoprost, the most widely prescribed prostaglandin analogue, and netarsudil, the active ingredient in Rhopressa (netarsudil ophthalmic solution 0.02%), a first-in-class rho-kinase inhibitor specifically designed to target the trabecular meshwork.
Dexycu (EyePoint Pharmaceuticals)
EyePoint Pharmaceuticals commercially launched Dexycu (dexamethasone intraocular suspension 9%) in the United States. Dexycu is the first and only intraocular steroid approved by the FDA for the treatment of postoperative inflammation. It is administered as a single dose at the end of cataract surgery.

Avaclyr (Fera Pharmaceuticals)
Fera Pharmaceuticals received FDA approval for Avaclyr (acyclovir ophthalmic ointment 3%) for the treatment of herpetic keratitis. Orphan drug exclusivity was also granted, providing 7 years of marketing exclusivity for the product.
February 2019
Lotemax SM (Bausch + Lomb)
Bausch + Lomb received FDA approval for Lotemax SM (loteprednol etabonate ophthalmic gel 0.38%), a gel formulation for the treatment of postoperative inflammation and pain following ocular surgery. Compared to Lotemax Gel 0.5%, Lotemax SM delivers a submicron particle size for faster drug dissolution in tears. Lotemax SM also provides two times greater penetration to the aqueous humor compared to Lotemax Gel.

Yutiq (EyePoint Pharmaceuticals)
EyePoint Pharmaceuticals announced the commercial launch of Yutiq (fluocinolone acetonide intravitreal implant 0.18 mg), a 3-year microinsert for the treatment of chronic noninfectious uveitis affecting the posterior segment of the eye. Yutiq utilizes the company’s Durasert drug delivery technology and is an intravitreal microinsert containing 0.18 mg of fluocinolone acetonide, designed to release the drug consistently for up to 36 months. Yutiq is supplied in a sterile single-dose preloaded applicator that can be administered in the physician’s office.
January 2019
Inveltys (Kala Pharmaceuticals)
Kala Pharmaceuticals launched Inveltys (loteprednol etabonate ophthalmic suspension 1%), the first and only twice-daily ocular corticosteroid indicated for the treatment of postoperative inflammation and pain following ocular surgery. Kala also announced the hiring of specialty ophthalmology sales organization. Inveltys is now in national and regional pharmaceutical distribution centers, and patients have access to Inveltys through their local retail pharmacies.
OPHTHALMIC DEVICES
October 2019

iDesign Refractive Studio New Indication (Johnson & Johnson Vision)
Johnson & Johnson Vision received FDA approval of a wavefront-guided PRK indication for the iDesign Refractive Studio, making it the first and only system with such an indication. The software will be commercially available in the first quarter of 2020.

Tecnis Simplicity Delivery System (Johnson & Johnson Vision)
Johnson & Johnson Vision announced the FDA approval and launch of the Tecnis Simplicity Delivery System, a preloaded, disposable IOL delivery system that is designed to prevent loading errors, simplify lens delivery, and protect against contamination when paired with a Tecnis one-piece IOL.

Silverstone Swept Source OCT (Optos)
Optos launched the Silverstone, which combines ultra-widefield retinal imaging with integrated, image-guided, swept-source OCT. The device combines color, autofluorescence, fluorescein, and indocyanine green angiography with swept-source OCT imaging capabilities.

Leaf Green Laser Photocoagulator (Norlase)
Norlase received FDA 510(k) clearance and announced the commercial launch of Leaf, which allows ophthalmologists to perform laser therapy in almost any exam room with minimal setup time and physical space. The entire laser unit attaches to an existing slit lamp, eliminating the need for a cart or counter space to house the laser console.
September 2019

Aquariuz Ablation Laser (Ziemer)
At the ESCRS Annual Meeting in Paris, Ziemer displayed for the first time the Aquariuz ablation laser, a compact solid state ablation laser for refractive surgery. The Aquariuz can be integrated with the company’s femtosecond lasers (Femto LDV Z-Models) and diagnostic devices (Galilei G-Models).

WaveLight Refractive Suite Upgrades (Alcon)
Alcon announced two additions to its WaveLight Refractive Suite. The Phorcides Analytic Engine diagnostic software, designed to help analyze and confirm the topographic treatment for an eye in Contoura Vision, allows surgeons to calculate optimal sphere and cylinder treatment. The Easypack Patient Interface optimizes outcomes and further streamlines efficiencies during LASIK surgery.
Cirrus 6000 OCT System (Carl Zeiss Meditec)
Carl Zeiss Meditec launched the Cirrus 6000 OCT system. Its 100-kHz speed allows clinicians to scan patients faster, with increased efficiency and improved imaging detail. Other features include wider and deeper OCT and OCT angiography (OCTA) scans with 12 x 12 mm OCTA and B-scan depth up to 2.9 mm; an HD AngioPlex scan; new workflow protocols to aid in increasing efficiency; and a wellness report to help educate patients.

EQ Workplace (Carl Zeiss Meditec)
The EQ Workplace offers surgeons a digital solution to connect and streamline their refractive cataract workflow. From biometry and calculating, selecting and ordering IOLs to surgical planning and postoperative data collection, Carl Zeiss Meditec said the EQ Workplace saves time during preoperative processes.
miLoop (Carl Zeiss Meditec)
Carl Zeiss Meditec also announced that it received the CE Mark for the miLoop, a microinterventional device designed to deliver zero-energy endocapsular lens fragmentation. Using microthin, super-elastic, self-expanding nitinol filament technology, the miLoop is designed to offer cataract surgeons the ability to achieve full-thickness lens fragmentation for any grade cataract, minimizing intraocular ultrasonic vibrations and phaco power.
Eva Upgrade Enhancements (Dutch Ophthalmic)
Dutch Ophthalmic obtained 510(k) clearance for a range of enhancements to its Eva surgical system, including a redesigned footpedal that allows the Eva endolaser to be integrated into the main footpedal, providing seamless switching from vitrectomy to laser and eliminating the need for a secondary laser pedal; and an improved LED light source that provides increased illumination with smaller gauge fibers.

Ellex Eye Prime (Haag-Streit)
Haag-Streit UK launched the Eye Prime diagnostic ultrasound system in the United Kingdom. The Eye Prime features six-ring phased annular array technology, which the company says delivers crisp, high-definition images and focal point accuracy.
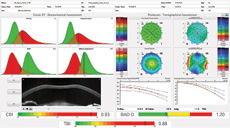
Corvis ST Updates (Oculus)
Oculus released software updates for the Corvis ST tonometer that allow the assessment of biomechanical stability after corneal refractive surgery, including a comparison display that allows monitoring biomechanical changes over time; the quantification of corneal elasticity based on the stress-strain behavior of corneal tissue; and glaucoma screening.

EyeKinetix Pupillograph (Konan Medical)
Konan Medical announced that its second-generation pupillograph, EyeKinetix, is FDA-listed and available for sale in the United States. EyeKinetix is smaller, faster, and less expensive than its predecessor, RAPDx.
Cassini Updates (Cassini Technologies)
Cassini Technologies revealed its latest version of the Cassini device, which features a new design, camera technology, and improved patient comfort and usability. It also uses reduced illumination during measurements.

Atos Femtosecond Laser With SmartSight (Schwind eye-tech-solutions)
Schwind eye-tech-solutions introduced Schwind Atos, a femtosecond laser that features an advanced minimally invasive lenticule extraction procedure called SmartSight. Featuring intelligent eye tracking with pupil recognition and cyclotorsion compensation, the laser system provides precise centering of the patient’s eye along the visual axis. SmartSight is suitable for the treatment of myopia and astigmatism up to 5.00 D.
Scleral IOL Fixation Solutions Pack (MicroSurgical Technology)
MicroSurgical Technology introduced the Scleral IOL Fixation Solutions Pack, which is designed to streamline the double-needle scleral IOL fixation technique. The pack includes several unique instruments that are packaged to support the procedure end-to-end.
August 2019

Pentacam AXL Wave (Oculus)
Oculus introduced its next-generation Pentacam—the Pentacam AXL Wave—which is the first device to combine Scheimpflug tomography with axial length, total eye wavefront, objective refraction, and retroillumination, according to the company. New in the Pentacam AXL Wave is wavefront aberrometry of the entire eye and a retroillumination technology for preoperative assessment of crystalline lens opacities and postoperative monitoring of IOL position.

Myopia Master (Oculus)
The company also introduced the Myopia Master, a device designed to combine important measurement methods for myopia management: axial length, refraction values, and the central corneal radii. The Myopia Master creates a myopia report for each patient, giving consideration to such myopia risk factors as having myopic parents, time spent outdoors, and time spent on near-vision activities. The device can be mounted on a workstation or on an ophthalmic table.
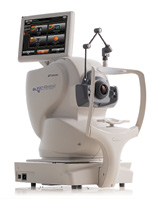
Maestro2 OCT/Fundus Camera (Topcon)
Topcon launched the Maestro2 Automated OCT/Fundus Camera with OCTA. The Maestro2 is a fully automated OCT system that can capture high-resolution nonmydriatic, true color fundus photography, OCT, and OCTA with the single press of a button. The multimodal system also offers the Hood Report for the structural and functional analysis of glaucoma.
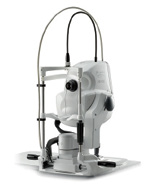
Mirante Scanning Laser Ophthalmoscope (Nidek)
Nidek launched the Mirante Scanning Laser Ophthalmoscope, a multimodal fundus imaging platform that combines high-definition scanning laser ophthalmoscopy and OCT with ultra-widefield imaging. The multimodal platform captures high-quality color images, fluorescein angiography, indocyanine green angiography, fundus autofluorescence, unique retro-mode images, OCT, and OCTA.
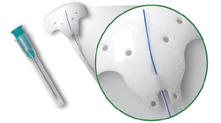
Ahmed ClearPath Glaucoma Drainage Device (New World Medical)
New World Medical launched the Ahmed ClearPath glaucoma drainage device, which is available in two sizes, 350 and 250 mm2. The Ahmed ClearPath implant is a flexible plate with a contour that closely conforms to the curvature of the eye. The suture fixation points are positioned more anteriorly on the device compared to other valveless drainage devices.
July 2019
Clarus 700 (Carl Zeiss Meditec)
Carl Zeiss Meditec received FDA 510(k) clearance for the Clarus 700 high-definition, ultra-widefield imaging system. Clarus 700 is the first high-resolution ultra-widefield imaging device with true color and a complete range of fundus imaging modalities, including fluorescein angiography, according to the company.

MST 19-Gauge Ahmed Micro Stent Cutter (MicroSurgical Technology)
MicroSurgical Technology, in collaboration with glaucoma and cataract surgeon Iqbal Ike K. Ahmed, MD, FRCSC, developed the 19-gauge Ahmed Micro Stent Cutter, which enables an efficient and minimally invasive solution for trimming stent protrusions.
June 2019

LacryStim IPL (Quantel Medical)
Quantel Medical received the CE Mark for the LacryStim IPL system for the treatment of dry eye diseases. LacryStim IPL features a unique wavelength spectrum and train of pulses enabling the stimulation of the lachrimal and meibomian glands and the reduction of inflammation. These mechanisms of action help improve the tear film quality and reduce the major symptoms associated with mild to moderate dry eye disease.
May 2019

YC-200 S Plus/YC-200 YAG Laser (Nidek)
Nidek launched the YC-200 S Plus ophthalmic Nd:YAG and selective laser trabeculoplasty laser system YC-200 ophthalmic Nd:YAG laser system in Europe. The YC-200 S Plus/YC-200 builds on the technology of the YC-1800 laser and incorporates new optical designs, engineering, and software advances to ensure precise targeting of pathology, while ensuring efficacious treatments and enhancing surgeon visualization of laser delivery, according to Nidek.
April 2019
TearCare (Sight Sciences)
Sight Sciences commercially launched the TearCare dry eye treatment device. TearCare allows the patient’s eyes to remain open and blinking during a procedure in which localized heat application is needed. Soft, flexible thermal devices conform to the eyelids to deliver a therapeutic level of heat for a specific period of time to soften and liquefy meibum, which prevents tear film evaporation.

Plex Elite 2.0 (Carl Zeiss Meditec)
Carl Zeiss Meditec launched the Plex Elite 2.0, a dual-speed swept-source OCT/OCTA that will scan at 200 and 100 kHz, providing a more detailed view into the retina. Varying speeds can be applied to different disease states in the eye.

Faros Compact Surgical Platform Upgrade (Oertli)
Oertli Instrumente upgraded its compact surgical platform Faros with features aimed at making the surgeon’s work easier, safer, and more efficient. The Faros features a two-pump system with flow and vacuum control. The instrument connections are immediately accessible from the front, speeding up surgical procedures.

RT-6100 Refractor and TS-610 Tabletop Refraction System (Nidek)
Nidek launched the RT-6100 Intelligent Refractor, which provides a streamlined refractor head and user-friendly control console for precise and quick examinations. The RT-6100 also comprises part of the TS-610 Tabletop Refraction System, a subjective refraction workstation that integrates the chart and refractor into a single unit.
March 2019
ABSolu Ultrasound Platform (Quantel Medical)
Quantel Medical received FDA approval for the A/B/S ultrasound platform: ABSolu. The ABSolu includes a five-ring annular technology 20-MHz B probe that increases the depth of field by 70%, offering high-definition information of the vitreous, retinal wall, and orbit in a single scan.

Maestro Unlimited OCT System (Topcon)
Topcon unveiled the Maestro Unlimited, which pairs the Maestro 3D OCT with the Topcon Harmony, a web-based data management application that provides unlimited device connections within a practice. The integration of the two products gives eye care a fully automated OCT/fundus camera imaging system with a secure platform to store and archive patient examination data.
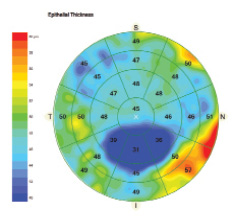
Cirrus Epithelial Thickness Mapping (Carl Zeiss Meditec)
Carl Zeiss Meditec received FDA 510(k) clearance for the Cirrus HD-OCT platform, expanding the capabilities of its Anterior Segment Premier Module to include epithelial thickness mapping (ETM). ETM with Cirrus provides a detailed 9-mm map of epithelial thickness that enables more thorough assessment of patients before refractive surgery, allows monitoring of the cornea’s response to treatment, and aids in managing patients with ocular surface disorders and progressive corneal diseases.
February 2019

Functional Vision EyeQ (RightEye)
RightEye launched Functional Vision EyeQ, an automated test that allows optometrists to objectively identify functional vision issues that affect a host of everyday activities. Once a problem is identified, Functional Vision EyeQ recommends computer-based exercises for patients to do at home, under the supervision of their optometrist.
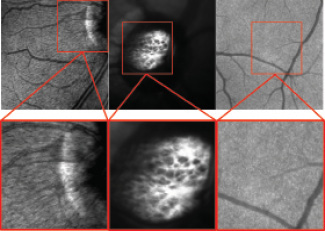
Spectralis High Magnification Module (Heidelberg Engineering)
Heidelberg Engineering received the CE Mark for the Spectralis High Magnification Module. With the addition of a lens and software upgrade, the module enables visualization of the ocular fundus at a microscopic level.
IOLs
September 2019

Tecnis Synergy IOL (Johnson & Johnson Vision)
At the ESCRS meeting in Paris, Johnson & Johnson Vision announced the availability of the Tecnis Synergy IOL in Europe, Australia, and New Zealand. According to the company, the Tecnis Synergy provides a broad range of continuous vision, covering from distance to 33 cm; eliminates the visual gaps present in trifocal and other multifocal technology; continues to deliver superior performance in low-light conditions; and features violet-filtering technology demonstrating reduction in halo intensity.

FineVision Triumf Trifocal EDOF IOL (PhysIOL)
Also at ESCRS, BVI, through its PhysIOL subsidiary, launched the FineVision Triumf extended depth of focus (EDOF) trifocal presbyopia-correcting IOL in Europe. The FineVision Triumf combines trifocal technology and EDOF optics with the goal of reducing low light side effects such as glare and halos experienced by some patients after implantation of trifocal IOLs.

xact Mono-EDoF IOL (Santen)
Santen launched the xact Mono-EDoF IOL in Europe during the 2019 ESCRS meeting. Santan says the xact provides excellent visual acuity at far distances as expected from a monofocal IOL, but additionally offers functional visual acuity at intermediate distances thanks to its novel continuous ranges of focus optical design, with no significant visual side effects.
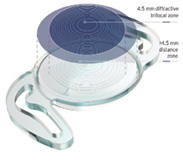
RayOne Trifocal Toric (Rayner)
Rayner launched its RayOne Trifocal Toric fully preloaded IOL. With 11% light loss from its patented diffractive design, RayOne Trifocal Toric gives cataract patients with varying degrees of preoperative corneal astigmatism the opportunity to become spectacle independent.
August 2019
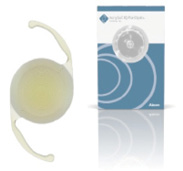
AcrySof IQ PanOptix Trifocal IOL (Alcon)
Alcon received FDA approval for and commercially launched the AcrySof IQ PanOptix Trifocal IOL, the first and only trifocal lens approved in the United States. The PanOptix Trifocal, which is available in spherical and toric designs, uses Enlighten Optical Technology, a proprietary design that optimizes intermediate vision without compromising near and distance vision.
July 2019
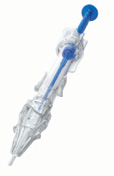
Nanex multiSert+ IOL Delivery System (Hoya Surgical Optics)
Hoya Surgical Optics obtained the CE Mark for Nanex multiSert+, which consists of an IOL delivered in a first-of-its-kind preloaded delivery system. The 1.62-mm injector tip of multiSert+ is the world’s smallest nozzle size in an open-loop preloaded hydrophobic IOL system, designed for microincision cataract surgery as low as 1.8 mm, according to Hoya.
February 2019

Tecnis Eyhance IOL (Johnson & Johnson Vision)
Johnson & Johnson Vision launched the Tecnis Eyhance IOL in Europe. The Tecnis Eyhance is a monofocal IOL that allows patients to achieve significantly improved intermediate vision compared with a standard aspheric monofocal IOL, along with 20/20 distance vision, according to the company.
January 2019

Zen Multifocal Scleral Lens (Bausch + Lomb)
Bausch + Lomb introduced the Zen multifocal scleral lens for presbyopia, which will be exclusively available with Zenlens and Zen RC scleral lenses. The Zen multifocal scleral lens will allow eye care professionals to fit presbyopic patients who have irregular and regular corneas, as well as those who have ocular surface disease such as dry eye, with add power ranges from +1.00 to +3.50 D in 0.25 D steps.
1. Cunningham E. Strategic Transactions | Pharma Intelligence, 2019. Paper presented at: the Ophthalmology Innovation Summit; San Francisco; October 10, 2019.




