Every day that we prepare in the operating room, we want to be our best for the patients who put their trust in us. When technologies come along that allow us to improve, we certainly welcome their help.
With these ideas in mind, I have recently begun using the CENTURION Vision System (Alcon) new ACTIVE SENTRY Handpiece (Alcon), which has built-in sensors that immediately detect changes in the anterior chamber and trigger a faster response from the fluidics system. I’ve also started using the system’s INTREPID Hybrid Tip (Alcon), a polymer phaco tip that reduces the risk of capsular damage. Individually, the two technologies have made a measurable difference in my cataract surgery; together, they help improve safety and efficiency on several levels.
Active Sentry Stability
Cataract surgery is the most common surgical procedure performed in the United States, and it is generally safe. I welcome every incremental improvement we can make. ACTIVE SENTRY is a meaningful evolution that’s taking an already good surgery and making it better.
The purpose behind the ACTIVE SENTRY is to move sensors from the phaco unit (Active Fluidics, Alcon) to the handpiece, making the system more responsive and the chamber more predictably stable. Not only does this help patients with a known risk of instability, but I think performing surgery with the ACTIVE SENTRY helps in every case.
My dad always said, “Routine surgery only happens to other people.” We don’t always know that an eye will have a problem until the problem actually occurs. It’s always best to prevent a problem and avoid a surprise, and ACTIVE SENTRY does that in the most unobtrusive way.
That said, the cases where I rely heavily on the ACTIVE SENTRY advantages are situations where the zonules might be loose. I want that chamber to be absolutely rock-solid steady, with vitreous stability and no bag popping up and down. In these cases, I have a much-improved surgical experience.
The Experience of Using Active Sentry
When I learned about the ACTIVE SENTRY Handpiece, the concept felt natural to me. I knew I would love to have less surge and less lag time in the fluidics adjustment. It made sense to me to have an IOP sensor in the handpiece, where it’s closer and the system can react quicker. I didn’t feel any skepticism, but I was curious to see if the chamber would be noticeably more stable and if I would feel it when the ACTIVE SENTRY actuates.
For me, one of the markers of a fantastic technology is that the first time I use it, it feels like I’ve always used it. I quickly found that the ACTIVE SENTRY Handpiece felt even more natural than other handpieces I’d used. Having the IOP sensor in the handpiece, reacting quicker, and reducing the lag time that I was accustomed to for fluidics to correct the IOP felt very natural as well.
When I used the ACTIVE SENTRY, the chamber was more stable, but in most cases I didn’t notice it. It felt like my procedures were uneventful. I did note the added chamber stability in floppy iris and loose zonule cases.
Sometimes, we realize how much we liked something when it’s taken away. When I tried going back to using the standard handpiece, I immediately noticed that I’d been taking the ACTIVE SENTRY Handpiece for granted. ACTIVE SENTRY had been working behind the scenes to make sure my surgery would go smoothly. I could not tell when ACTIVE SENTRY activated during surgery, but after each case, I would look at the metrics and see the technology engaged 5 to 10 times to prevent surge during surgery. Now I will use ACTIVE SENTRY for all my cases—there’s no reason not to.
Added Safety of the INTREPID Hybrid Tip
The polymer INTREPID Hybrid Tip for the phaco needle is the same tip material that I’ve grown to love in the Polymer I/A (Alcon). When I learned it would be available in the phaco needle, I thought it sounded like a natural extension of that technology, but I was also concerned that it might inhibit my cutting efficiency and increase my energies during surgery.
I found that using the Hybrid Tip felt very much like using a metal tip, but with added safety. I use it to take out virtually any type of cataract, grades 1 through 3. I suspect that it would be possible to use the Hybrid Tip on even denser cataracts, but I would need more experience first. I think the cutting efficiency of a traditional INTREPID Balanced Tip would likely be a better option for very dense cataracts, where we want every bit of cutting efficiency we can get.
What I love about the Hybrid Tip is the added safety it provides. If we inadvertently grab the capsular bag, we will not end up in a difficult spot. That makes the tip one of those really nice additions that feels good and is easy to use, and it makes cataract surgery incrementally safer. In my OR, we’re also training fellows. When the phaco tip they’re using is the Hybrid Tip, I feel much more comfortable supervising surgery. That extra level of safety means we worry less about incidents during training.
Combined Benefits of Both New Technologies
When we take the CENTURION Vision System platform and add ACTIVE SENTRY technology, we get a more stable anterior chamber, which is safer and also further streamlines the procedure. Now we can also use the INTERPID Hybrid Tip for phacoemulsification, which lets us work more freely and efficiently. Both are incremental changes that make an already safe procedure even safer and more efficient. I feel good that we have partners in the industry who are continually raising the bar for the safety and quality of important vision-saving surgeries. When I prepare to give my best every day in the OR, advances like these help me achieve the best results for my patients.
Case: Loose Lens with Active Sentry and Hybrid Tip
A 72-year-old patient presented with a grade 2 to 3 cataract. The patient’s refraction was myopic, and we determined that a standard IOL was the best choice to deliver the visual outcomes he wanted. The eye exhibited some phacodonesis upon initiating the capsulotomy, so anterior chamber stability was a concern.
INTREPID Hybrid Tip: This was quite a thick lens, and the INTREPID Hybrid Tip cut through it with beautiful efficiency. At the same time, I felt very confident that the polymer tip would make cutting safer. I had more courage to take the Hybrid Tip a little farther posteriorly on my groove because I felt like, if I inadvertently grabbed that posterior capsule, it would be much less likely to tear. It was problem-free (Figure 1).
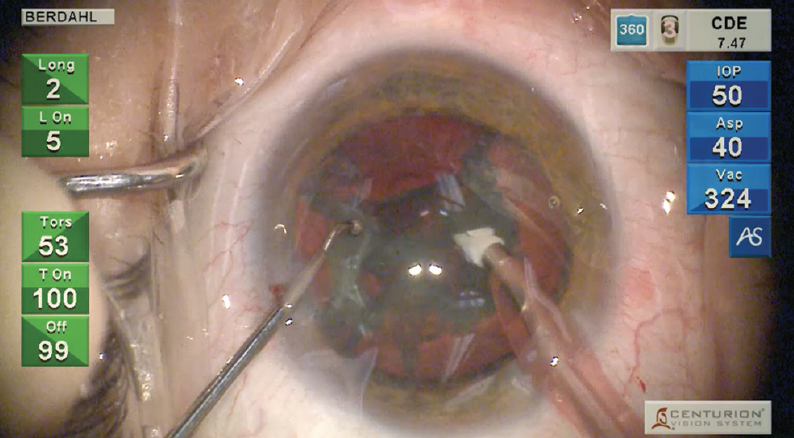
Figure 1. I used the INTREPID Hybrid Tip to break up a thick cataract. The safety of the tip allowed me to move farther posteriorly with minimal concern about damage to the capsular bag.
ACTIVE SENTRY Handpiece and Anterior Chamber Stability: The lens came out very nicely. While I was removing this rather meaty cataract, the anterior chamber remained very stable. That’s especially important when we have a lens like this one, which seemed a little on the loose side.
With the ACTIVE SENTRY continually monitoring and prompting adjustments from the Active Fluidics system, there was less bounce of the posterior capsule up to my tip. If that had bounced up, the Hybrid Tip would have helped mitigate the risk of a tear to the posterior capsule.
ACTIVE SENTRY Metrics: The ACTIVE SENTRY handpiece actuated 11 times during surgery, prompting adjustments from the Active Fluidics system. This number is on the higher side. What that means is that 11 times during surgery, the chamber was going to become unstable, but the ACTIVE SENTRY reacted instantly, so the chamber appeared to be stable throughout the procedure (Figure 2).
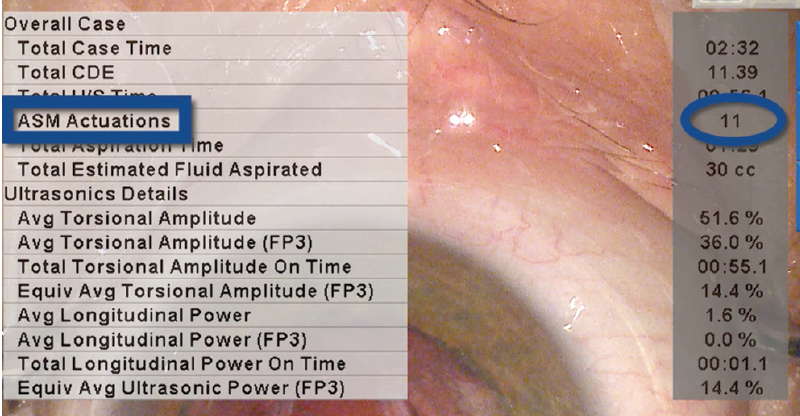
Figure 2. The ACTIVE SENTRY Handpiece actuated 11 times during surgery. Each time the chamber was going to become unstable, the system reacted instantly, prompting adjustments from the Active Fluidics system to keep the chamber stable throughout the procedure. This process was invisible to me as a surgeon.
ACTIVE SENTRY work is invisible to me because it happens so quickly. Within milliseconds, the sensor determines that the anterior chamber eye pressure isn’t where we set it, and Active Fluidics pushes fluid into the eye to make sure the chamber is pressurized properly. These are my favorite kind of problems—the ones that a machine solves for me before I even know they exist.
Capsule tension ring (CTR) and lens placement: At the beginning of the case, I could tell that there was some looseness of the capsular bag, so at this point I slowed down and put in a CTR for better centration and stability. CTRs are very effective when there’s a peripheral zonulopath. I like to insert these rings in a very tangential way so that they just slide in with almost no pressure on the zonules. I was able to place the IOL with no trouble. The lens was well centered, and the addition of the CTR mitigated the capsule instability.
A safer procedure: In this case, I think that combining the Hybrid Tip with the ACTIVE SENTRY Handpiece helped diminish the pressure fluctuation and maintain a safer situation for this patient. We never know when we might get into trouble, but I love knowing that I’m limiting that possibility a bit more with these surgical technologies. The patient’s outcomes were excellent, with a mild prescription producing 20/20 BCVA.
CENTURION® Vision System Important Product Information
Federal (USA) law restricts this device to sale by, or on the order of, a physician.
As part of a properly maintained surgical environment, it is recommended that a backup IOL injector be made available in the event the AutoSert® IOL Injector Handpiece does not perform as expected.
INDICATION: The CENTURION® Vision system is indicated for emulsification, separation, irrigation, and aspiration of cataracts, residual cortical material and lens epithelial cells, vitreous aspiration and cutting associated with anterior vitrectomy, bipolar coagulation, and intraocular lens injection. The AutoSert® IOL Injector Handpiece is intended to deliver qualified AcrySof® intraocular lenses into the eye following cataract removal.
The AutoSert® IOL Injector Handpiece achieves the functionality of injection of intraocular lenses. The AutoSert® IOL Injector Handpiece is indicated for use with the AcrySof® lenses SN6OWF, SN6AD1, SN6AT3 through SN6AT9, as well as approved AcrySof® lenses that are specifically indicated for use with this inserter, as indicated in the approved labeling of those lenses.
WARNINGS: Appropriate use of CENTURION® Vision System parameters and accessories is important for successful procedures. Use of low vacuum limits, low flow rates, low bottle heights, high power settings, extended power usage, power usage during occlusion conditions (beeping tones), failure to sufficiently aspirate viscoelastic prior to using power, excessively tight incisions, and combinations of the above actions may result in significant temperature increases at incision site and inside the eye, and lead to severe thermal eye tissue damage.
Good clinical practice dictates the testing for adequate irrigation and aspiration flow prior to entering the eye. Ensure that tubings are not occluded or pinched during any phase of operation.
AES/COMPLICATIONS:Inadvertent actuation of Prime or Tune while a handpiece is in the eye can create a hazardous condition that may result in patient injury. During any ultrasonic procedure, metal particles may result from inadvertent touching of the ultrasonic tip with a second instrument. Another potential source of metal particles resulting from any ultrasonic handpiece may be the result of ultrasonic energy causing micro abrasion of the ultrasonic tip.
ATTENTION: Refer to the Directions for Use for the accessories/consumables and Operator’s Manual for a complete listing of indications, warnings, cautions and notes
Important Product Information for DisCoVisc® OVD
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
INDICATIONS: DisCoVisc® (Sodium Chondroitin Sulfate-Sodium Hyaluronate) Ophthalmic Viscosurgical Device (OVD) is indicated for use during surgery in the anterior segment of the eye. It is designed to create and maintain space, to protect the corneal endothelium and other intraocular tissues and to manipulate tissues during surgery. It may also be used to coat intraocular lenses and instruments during cataract extraction and IOL insertion.
WARNINGS/PRECAUTIONS: Failure to follow assembly instructions or use of an alternate cannula may result in cannula detachment and potential patient injury. Precautions are limited to those normally associated with the surgical procedure being performed. Although sodium hyaluronate and sodium chondroitin sulfate are highly purified biological polymers, the physician should be aware of the potential allergic risks inherent in the use of any biological material.
ADVERSE REACTIONS: DisCoVisc® Ophthalmic Viscosurgical Device was very well tolerated in nonclinical and clinical studies. A transient rise in intraocular pressure in the early postoperative period may be expected due to the presence of sodium hyaluronate, which has been shown to affect such a rise. It is therefore recommended that DisCoVisc® OVD be removed from the anterior chamber by thorough irrigation and/or aspiration at the end of surgery to minimize postoperative IOP increases.
ATTENTION: Reference the Directions for Use for a complete listing of warnings and precautions.
Important Product Information for PROVISC® OVD
CAUTION: Federal (USA) law restricts this device to sale by, or on the order of, a physician.
INDICATION: PROVISC® (Sodium Hyaluronate) Ophthalmic Viscoelastic Device (OVD) is indicated for use as an ophthalmic surgical aid in the anterior segment during cataract extraction and intraocular lens (IOL) implantation. Ophthalmic viscoelastics serve to maintain a deep anterior chamber during anterior segment surgery allowing reduced trauma to the corneal endothelium and surrounding ocular tissues. They help push back the vitreous face and prevent formation of a flat chamber during surgery.
WARNINGS/PRECAUTIONS:
• Postoperative increases in intraocular pressure have been reported with sodium hyaluronate products. The IOP should be carefully monitored and appropriate therapy instituted if significant increases should occur. It is recommended that PROVISC® OVD be removed by irrigation and/or aspiration at the close of surgery. Do not overfill anterior chamber. Although sodium hyaluronate is a highly purified biological polymer the physician should be aware of the potential allergic risks inherent in the use of any biological material; care should be used in patients with hypersensitivity to any components in this material. Cannula assembly instructions should be followed to prevent patient injury.
ADVERSE EVENTS:
• Postoperative inflammatory reactions such as hypopyon and iritis have been reported with the use of ophthalmic viscoelastics, as well as incidents of corneal edema, corneal decompensation, and a transient rise intraocular pressure.
ATTENTION: Reference the directions for use for a complete listing of indications, warnings and precautions.




