CASE PRESENTATION
A 91-year-old woman is referred for treatment of a dislocated IOL in her right eye. The patient presents with a 6-week history of decreased vision in the right eye that she says is “like a curtain has come down.” She reports increased difficulty judging distances, watching TV, and painting with watercolors.
The patient has a history of cataract surgery on both eyes and pseudoexfoliation in the right eye. She instills a fixed combination of brinzolamide 1% and brimonidine 0.2% (Simbrinza, Alcon) three times daily in the right eye only, but IOP is poorly controlled at present. Her medical history is significant for hypertension, thyroid disease, hyperlipidemia, and coronary artery disease. The patient is currently using several medications, including aspirin, and her cardiologist prefers that she not stop the aspirin prior to surgery.
On examination, BCVA is -1.25 -1.00 x 110º = 20/40 OD and -1.75 -0.50 x 095º = 20/20 OS. The patient describes her vision as “coming in and out.” IOP is 32 mm Hg OD and 19 mm Hg OS. Confrontation visual fields show a nasal defect in the right eye. The dislocated three-piece IOL in the right eye exhibits significant mobility with eye movement. A posterior segment examination shows a cup-to-disc ratio of 0.6 in the right eye and 0.1 in the left. A few drusen are present in the retinal periphery of each eye. OCT imaging shows temporal thinning of the retinal nerve fiber layer in the right eye and normal thickness in the left eye.
What surgical options are available to treat this patient? Are you concerned about not stopping aspirin therapy prior to surgery? Does this influence your treatment choices?
—Case prepared by Cathleen M. McCabe, MD

GARRY P. CONDON, MD
Despite the elevated IOP, I would consider performing IOL fixation alone, without concurrent glaucoma surgery, as my initial approach to this elderly anticoagulated but symptomatic woman. Recent studies have suggested an associated IOP increase with in-the-bag IOL subluxation and a significant decrease in IOP after IOL fixation—a change that is even more pronounced in exfoliative glaucoma.1-3 Assuming that the IOL is in the bag, I would use a minimally invasive single-suture technique I have previously described to stabilize the IOL and minimize bleeding risk.4
For this technique, I use a single modified McCannel suture to fixate the superior capsulorhexis margin to the posterior aspect of the superior iris. I make corneal paracentesis tracks at the 10 and 2 clock positions. I inject a cohesive OVD into the anterior chamber and behind the upper iris to stabilize the anterior chamber, create space behind the superior iris, and tamponade the vitreous face.
Using microforceps via the nasal paracentesis, I grasp the edge of the fibrotic capsulorhexis while guiding a 10-0 polypropylene suture on a long, curved needle (Prolene, Ethicon) through the temporal paracentesis and directing it down through midperipheral iris 1 clock hour away from the 12-o’clock meridian. After piercing the iris, I rotate the tip of the needle and pass it through the capsular bag at the edge of the capsulorhexis while stabilizing the bag with the microforceps (Figure 1). I then release the edge of the capsulorhexis from the forceps and turn the needle back superiorly so that its tip passes over the same edge of the capsulorhexis (Figure 2). Next, I direct the needle up through midperipheral iris 2 clock hours nasal to the first iris entry point. Once the needle passes through this location in the iris, I push the needle out through clear cornea (Figure 3).
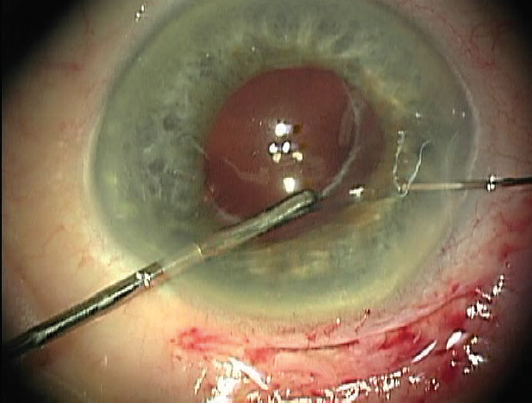
Figure 1. The needle is passed through cornea, mid-iris, and the edge of the capsulorhexis, which is held with microforceps.
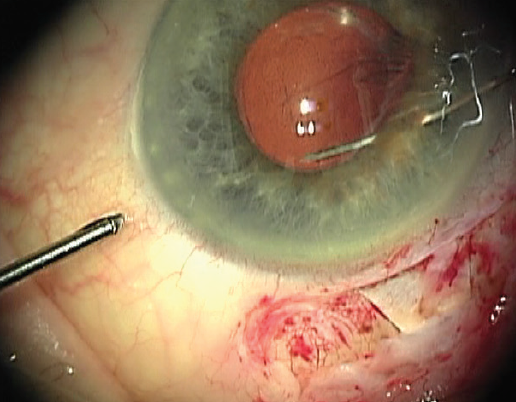
Figure 2. Once through the edge of the capsulorhexis, the needle is rotated back superiorly under the nasal iris.
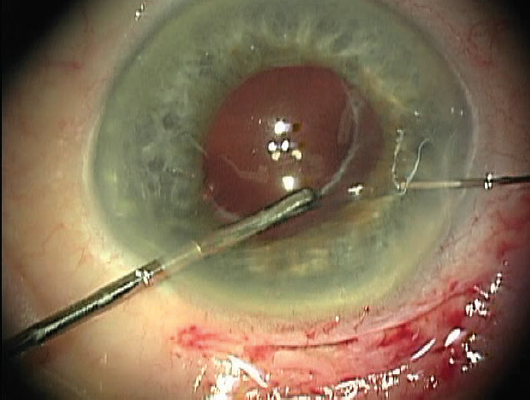
Figure 3. Passing the needle up through nasal iris and out through adjacent cornea completes the suture pass.
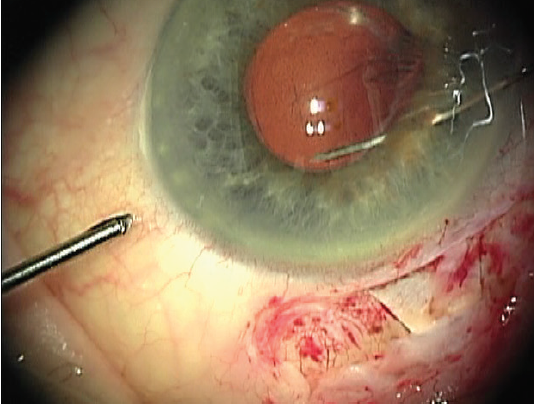
Figure 4. The finished suture fixes the capsulorhexis edge to the posterior surface of the iris.
Courtesy of Garry P. Condon, MD
A sliding Siepser knot secures the edge of the capsulorhexis to the posterior aspect of the iris, producing slight peaking of the pupillary margin (Figure 4). Alternatively, a double-armed suture via an inferior midperipheral paracentesis can accomplish the same fixation. A peripheral iridotomy intraoperatively or early postoperatively is required to avoid the pupillary block observed in some cases.
If fixation and medical therapy fail to control the IOP in this case, filtration surgery with a Xen Gel Stent (Allergan) is an option.

GILLES LESIEUR, MD
In some cases like this one, it is possible to preserve the capsular bag and secure the bag-IOL complex with a 9-0 polypropylene scleral stitch. For this patient with a highly mobile capsular bag, vision loss, and a curtain effect, my preference would be to explant the complex through a 5.5-mm incision under sideport infusion (anterior chamber maintainer) and then perform a vitrectomy. I would inject dexamethasone to remove all of the anterior vitreous and implant a Verisyse IOL (Johnson & Johnson Vision) in a posterior position. I would use local anesthesia for this case, specifically sub-Tenon anesthesia, as is my usual practice. Next, I would perform a posterior sclerotomy to treat the glaucoma in hopes of eliminating or at least minimizing the patient’s need for topical glaucoma therapy and decreasing the risk of disease progression.
Her use of aspirin does not worry me,5 so I would not suspend the treatment. I would, however, try to avoid any bleeding with positive pressure (anterior chamber maintainer) and by performing noninvasive glaucoma surgery such as nonpenetrating deep sclerectomy. I would also avoid placing a scleral stitch, which could pose a risk of bleeding for this patient.

BORIS MALYUGIN, MD, PHD
There are three main surgical options: (1) suture the existing IOL to the iris or sclera, (2) exchange the IOL for an anterior chamber IOL (ACIOL), or (3) exchange it for an iris-claw IOL. The first two options may lead to bleeding from the ciliary body or iris, whereas the third option will increase endothelial cell loss. The last option, also leading to partial blockage of the anterior chamber angle with IOL haptic elements, will further increase the chance of an IOP spike.
The case presentation does not describe the IOL model or disclose whether a capsular tension ring (CTR) is located inside the bag. If a CTR is in place, my preference would be two-point scleral fixation of the IOL–capsular bag–CTR complex with 9-0 polypropylene or PTFE (Gore-Tex, W.L. Gore & Associates, off-label use) sutures. The suture would circle the CTR, pass through the ciliary sulcus, and be fixated to the sclera.
If there is no CTR in the bag, my plan would be iris fixation with a 9-0 polypropylene suture. I would pass the long, curved needle through the paracentesis, puncture the iris and capsular bag, and then pass the needle around the IOL haptic, back to the anterior chamber, and out of the eye through clear cornea near the limbus. I would gently tie a Siepser sliding knot to avoid pupil ovalization. The second suture would be placed around the second haptic in a similar fashion.
The techniques of through-the-bag IOL suturing to the iris can be used with both one- and three-piece IOLs that have haptic elements, but they cannot be used with IOLs that have a one-piece monobloc design. With a monobloc lens, my preference would be to exchange it for an iris-claw IOL.
It is worth noting that the position of the lens may vary significantly depending on the patient’s position. For example, the IOL may look moderately displaced at the slit lamp but sink to somewhere near the equator under the operating microscope. The operating surgeon must therefore possess some vitreoretinal skills, including an ability to manipulate the posterior segment, as well as have access to proper equipment such as a vitrectomy machine and fundus lenses. That is why it is advisable to evaluate the IOL’s position preoperatively with ultrasound biomicroscopy while the patient is in a supine position. Doing so will help to avoid intraoperative surprises and changes to the surgical plan.
There are two main issues here, regardless of the surgical technique to be used: anticoagulation therapy and IOP control. I would not ask the patient to discontinue anticoagulants before ocular surgery because the bleeding can be controlled with an IOP increase, with an OVD, or with direct vessel cautery.
The second issue is high IOP (32 mm Hg) despite the use of several medications. That raises the question of whether to perform an IOP-lowering procedure after or at the time of IOL repositioning and refixation. I would prefer to perform refixation while the patient follows the maximum pressure-lowering medication regimen. Based on my experience, IOP is likely to decrease despite some risk of an early postoperative IOP spike, which should obviously be monitored and managed. That said, the possibility of a secondary IOP-lowering procedure should be discussed with the patient in advance.
A warning sign, however, is the patient’s complaint that “a curtain has come down.” The most likely cause of that curtain is the dislocated lens, but, to be on the safe side, I would rather rule out a retinal detachment with a careful fundus examination and B-scan ultrasonography.

MICHAEL PATTERSON, DO
The first step is to weigh the risks and benefits of surgery in this case. If surgery is elected, careful planning is necessary. It would be easy to choose to explant the IOL and then suture an IOL or fixate it to the sclera, but the patient’s age warrants consideration. If the cornea is healthy, the simplest course of action would be to remove the IOL and place an ACIOL.
I would place an anterior chamber maintainer and perform a two-port pars plana vitrectomy. This technique would allow me to remove the current IOL without destroying the retina by dragging vitreous around the posterior segment with the lens. I would use micrograsping forceps to hold the dislocated IOL in place prior to vitrectomy. After the vitrectomy, I would position the IOL in the anterior chamber, cut it, and remove it from the eye. It should then be easy to widen the temporal corneal incision and implant an ACIOL of appropriate size. I would place three sutures temporally in a slipknot fashion to minimize astigmatism and would expect a good refractive outcome. If one were concerned about corneal complications, I would recommend performing vitrectomy as detailed earlier, explanting the IOL, and then suturing the new IOL or using the Yamane technique.
I would consider performing selective laser trabeculoplasty prior to surgery to help manage the IOP. I would be disinclined to perform a microinvasive glaucoma surgery procedure because the case is already complex. If I did not perform selective laser trabeculoplasty, topical medication would likely lower the IOP sufficiently.
I have patients stop aspirin therapy only before full-thickness glaucoma surgery and oculoplastic procedures. I do not think there is any reason for this patient to stop aspirin therapy. Placing an anterior chamber maintainer to keep the eye inflated should decrease the risk of a suprachoroidal hemorrhage.

WHAT I DID: CATHLEEN M. MCCABE, MD
Late dislocation of an IOL is a known complication of cataract surgery in the setting of pseudoexfoliation. In this case, the IOL–capsular bag–cortical material complex dislocated inferiorly, resulting in intermittent blockage of the visual axis. Movement of the IOL was associated with IOP elevation, although no significant iris defects were observed. The patient’s anticoagulation with aspirin made decisions regarding the best approach to surgery more complex. To minimize bleeding, I decided not to perform a microinvasive or other glaucoma procedure. Another factor in my decision was that fixating a dislocated IOL can improve IOP control without further intervention, as Dr. Condon noted.
As with all complicated cataract and dislocated IOL surgeries, I discussed with the patient various surgical plans and explained that I would attempt to perform the least invasive, most straightforward surgery first and move on to other options as the situation dictated. Thinking through and preparing for several different surgical plans is critical to success in cases such as this one. My intention was to retain the current IOL but increase its stability, if possible. My first option was a new technique I have described for scleral fixation using 5-0 polypropylene intrascleral fixation with a flange.6 (For more details on this technique, see my contribution to the article, “Best Pearls of 2019,.) The backup plans were iris fixation with 10-0 polypropylene sutures and the Yamane technique of scleral fixation, which can necessitate an IOL exchange. Retaining the original IOL and capsular bag minimizes the need for an anterior vitrectomy, the loss of cortical material posteriorly, and the risk of damaging the endothelium during an IOL exchange.
I began by creating a paracentesis and filling the anterior chamber with a dispersive OVD. I cut a section of 5-0 polypropylene suture at a bevel and threaded it into a 27-gauge 1.25-in needle with the end exposed through the hub of the needle. Using an ink marker, I made paired marks 2 mm posterior to the limbus and 180º apart in the area of the haptics. I passed the 27-gauge needle through conjunctiva and sclera at the marked location, underneath the haptic, and through the capsular bag while using intraocular forceps as a guide. An assistant advanced the exposed end of the 5-0 polypropylene suture at the hub of the needle so that the suture protruded into the anterior chamber and could be grasped with intraocular forceps. The needle was withdrawn through the sclera and conjunctiva, and the polypropylene suture was grasped at the ocular surface.
I then passed a 27-gauge needle anterior to the first pass by 0.5 mm, this time entering the sulcus in front of the haptic-bag complex. Next I guided the needle end out of a paracentesis with forceps so that no corneal fibers were caught. The other end of the 5-0 polypropylene segment was then threaded into the externalized tip of the 27-gauge needle, the needle was withdrawn from the eye, and the exposed polypropylene suture was grasped at the ocular surface to create a belt loop of 5-0 polypropylene suture around the haptic-bag complex. The loop was cinched by slowly externalizing more of the suture.
The suture ends were trimmed and melted with low-temperature cautery to create a flange, as originally described by Yamane.7 Because the opposite haptic was not also supported by zonules, a similar procedure was performed 180º away to create a second belt loop around the fellow haptic. I placed a 23-gauge trocar and performed a limited anterior vitrectomy to remove vitreous from in front of the IOL and alleviate some posterior vitreous pressure. The IOL was centered and well supported at the end of the case.
I have used this technique to scleral-fixate dislocated lens-bag complexes with a variety of lens designs, including one-piece hydrophobic and hydrophilic acrylic IOLs, three-piece IOLs, and multifocal IOLs. Advantages of this technique include retention of the original lens (especially appreciated by my patients with multifocal or advanced technology lenses), less risk that cortical material will fall into the posterior segment, a stronger suture than smaller-diameter polypropylene (9-0) typically used for fixation, and often elimination of a need for an anterior vitrectomy. Long-term follow-up is necessary to determine the safety and longevity of this technique.
1. Bulnes BL, de Rojas Silva MV, Moore RL. Intraocular pressure changes before and after surgery for spontaneous in-the-bag intraocular lens dislocation. J Cataract Refract Surg. 2019;45(3):305-311.
2. Jakobsson G, Zetterberg M, Sundelin K, Stenevi U. Surgical repositioning of intraocular lenses after late dislocation: complications, effect on intraocular pressure, and visual outcomes. J Cataract Refract Surg. 2013;39(12):1879-1885.
3. Kristianslund O, Råen M, Østern AE, Drolsum L. Glaucoma and intraocular pressure in patients operated for late in-the-bag intraocular lens dislocation: a randomized clinical trial. Am J Ophthalmol. 2017;176:219-227.
4. Siegel MJ, Condon GP. Single suture iris-to-capsulorhexis fixation for in-the-bag intraocular lens subluxation. J Cataract Refract Surg. 2015;41(11):2347-2352.
5. Oh J, Smiddy WE, Kim SS. Antiplatelet and anticoagulation therapy in vitreoretinal surgery. Am J Ophthalmol. 2011;151(6):934-939.e3.
6. McCabe CM. Belt loop technique: a new method for scleral fixation of a dislocated IOL. Paper presented at: AECOS 2019 Summer Symposium; July 13, 2019; Deer Valley, Utah.
7. Yamane S. Transconjunctival intrascleral IOL fixation with double-needle technique. Film presented at: the ASCRS Symposium on Cataract, IOL and Refractive Surgery; May 6-10, 2016; New Orleans.




