
Lisa Brothers Arbisser, MD
Quieting the Eye
I am emeritus at my practice and no longer care for patients. If I did, however, I would use phenylephrine and ketorolac injection 1%/0.3% (Omidria, Omeros) in the infusion fluid for all complex cases—even for virtually every case. There is ample evidence that use of this product is associated with quieter eyes postoperatively and stable pupils intraoperatively.1 Especially with its pass-through status, and now with a permanent J code,2 it is accessible to our surgery centers and patients. The goal is to essentially hide from the immune system the inflammatory event of opening the lens capsule by suppressing expression of prostaglandins, especially when employing laser cataract surgery. I have, for years, made every effort with pre- and postoperative topical NSAIDs as well as postoperative nontapering topical steroids with rapid rehabilitation, clear corneas, thin maculas, and happy patients.
1. Walter K, Delwadia N, Coben J. Continuous intracameral phenylephrine-ketorolac irrigation for miosis prevention in femtosecond laser-assisted cataract surgery: Reduction in surgical time and iris manipulation. J Cataract Refract Surg. 2019;45(4):465-469.
2. Omeros’ Omidria Receives Product-Specific J-Code from CMS. https://eyewire.news/articles/omeros-omidria-receives-product-specific-j-code-from-cms/. Accessed October 18, 2019.

Steven J. Dell, MD
Mix-and-Match IOLs
For patient satisfaction and spectacle independence, the combination of a Symfony extended depth of focus (EDOF) IOL (Johnson & Johnson Vision) and a Tecnis Multifocal IOL (Johnson & Johnson Vision), with each eye set for distance, beats two Symfony IOLs with one eye set for mini-monovision. This was a conclusion of a paper presented by Jan Venter, MD, at this year’s ASCRS/ASOA Annual Meeting.1 In my practice, we have shifted toward this combination—mixing one Symfony and one Tecnis multifocal IOL—as a result.
1. Venter JA, Hannan SJ. Clinical and patient-reported outcomes of different combinations of diffractive and extended-depth-of focus IOLs. Paper presented at: the ASCRS/ASOA Annual Meeting; May 3-7, 2019; San Diego.

Uday Devgan, MD, FACS, FRCS
IOL Twist
There are times when an IOL exchange is needed in order to change dioptric power, to swap one optic design for another, or to replace a damaged or dislocated IOL. The most commonly used IOL model is a one-piece hydrophobic acrylic IOL, and these are available from most major ophthalmic manufacturers. The traditional method for explantation is to cut the IOL optic inside the anterior chamber, so that the 6-mm optic can be removed through a 3-mm incision. A better way is to explant the IOL without cutting it, using a method that I learned from Jack M. Chapman Jr, MD, of Gainesville, Georgia. This IOL twist technique involves rolling the IOL inside the anterior chamber, after which it can be explanted via an unenlarged 2.75-mm phaco incision.
In this procedure, the IOL is brought into the anterior chamber, which is filled with OVD, and one haptic is pulled through the phaco incision. Using straight tying forceps in the phaco incision, the optic is grasped at one side—not in the middle—while a spatula is introduced via the paracentesis incision. The spatula is placed over the optic to protect the corneal endothelium and to help roll the IOL optic. The forceps are rolled in the surgeon’s hand so that the IOL twists and becomes rolled onto itself. This compacted IOL can then be easily removed by simply pulling it out of the eye. Video demonstration of the IOL twist technique is viewable on www.cataractcoach.com or at bit.ly/devgan1219A.

Marjan Farid, MD
Treating Neurotrophic Keratitis
My top pearl of 2019 has been the ability to identify and treat neurotrophic keratitis (NK) earlier in the disease state. NK has traditionally been a challenging disorder to treat. We have been employing dry eye disease treatments to try to ameliorate and reverse NK with limited success. Addressing the underlying challenge of corneal nerve degeneration has been a challenge.
This year saw the approval of cenegermin-bkbj ophthalmic solution for topical ophthalmic use (Oxervate, Dompé) for the treatment of NK. With this recombinant human nerve growth factor, we can now target the underlying pathology of NK and administer meaningful treatment that lasts beyond immediate epithelial healing to achieve long-term sustained corneal clearance.
I have patients with severe punctate keratitis and epithelial irregularity without pain, as they are now correctly diagnosed with NK. Corneal sensation testing is becoming more routine in my corneal assessments because we now have a treatment that can address the underlying nerve issue. Patients with postherpetic neurotrophic corneas are another group that responds well to this treatment and can show long-lasting sustained healing of the epithelium after the 8-week course of cenegermin (Figure 1). My 2019 pearl is to check corneal sensation, identify NK, and treat it.
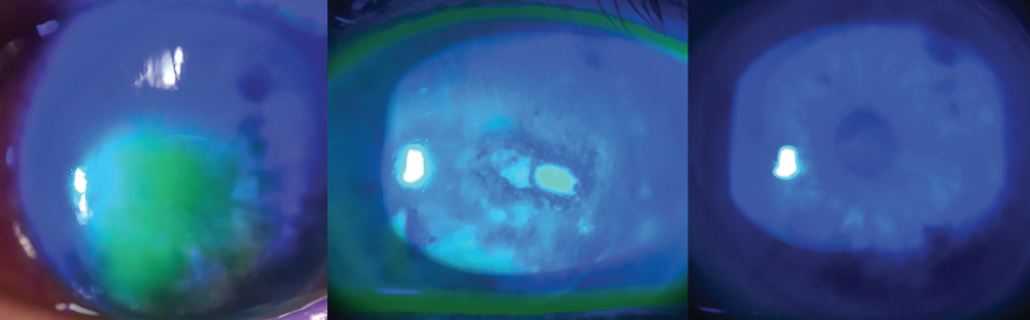
Figure 1. Baseline, week 4 of treatment, and week 8 of treatment (left to right) with cenegermin in a cornea with NK.
Courtesy of Marjan Farid, MD

Howard V. Gimbel, MD, MPH, FRCSC; and Hala Marzouk, MD
Haptic Tuck for Reverse Optic Capture
Reverse optic capture (ROC) techniques allow the implantation of a one-piece IOL in the event of a large posterior capsular tear occurring before lens placement. Haptic tuck is an alternative ROC technique that we and others find useful.
Toric IOLs and some multifocal IOLs are not available in three-piece IOL platforms. Surgery with the intent to implant a one-piece toric or nontoric IOL may be complicated by a large posterior capsular tear. In situations in which the tear occurs before the IOL is in the bag, a one-piece IOL may be injected through the continuous curvilinear capsulorhexis (CCC) and stabilized with a second instrument as the optic is pulled through the CCC for ROC, as described by Jones and colleagues.1 With this technique, however, there is risk of losing the IOL into the vitreous cavity when both haptics exit the injector cartridge before the optic edges can be captured to stabilize the IOL with ROC.
We previously described ROC for stabilizing a rotationally unstable toric IOL with the IOL already in the bag and the posterior capsule intact.2 This year, we published a new ROC technique for placing a one-piece acrylic IOL after a posterior capsular break.3 Instead of using the technique described by Jones et al, or replacing a planned toric one-piece IOL with a sulcus three-piece nontoric IOL for optic capture, we use a technique we call the haptic tuck, in which the planned toric IOL is used to achieve ROC.
In the haptic tuck technique, the toric IOL is placed in the sulcus and rotated to the planned axis (Figure 2). After more OVD is instilled if necessary, two instruments—a Sinskey hook and a spatula or two Sinskey hooks—are used to tuck one haptic at a time through the anterior CCC to capture the optic (Figure 3). Following successful ROC, the toric IOL may have to be rotated a few degrees to get the astigmatic axis perfectly aligned. The anterior chamber OVD is removed and the wounds are hydrated and confirmed to be watertight.
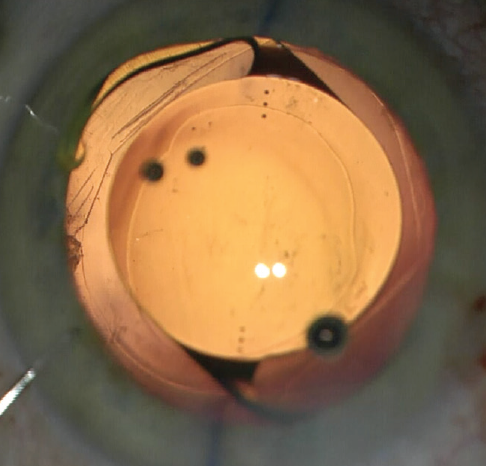
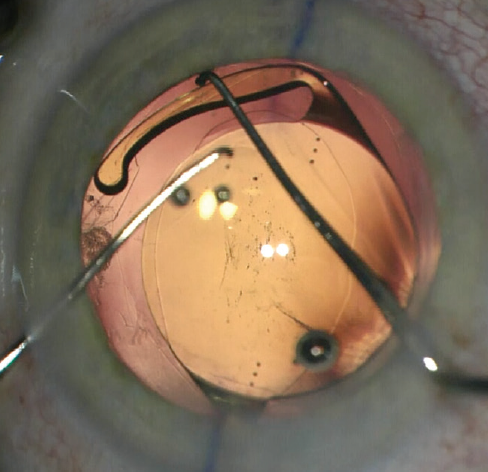
Figure 3. The edge of the optic is depressed to facilitate tucking the second haptic.
Courtesy of Howard Gimbel, MD, MPH, FRCSC
Although not as intuitive and conventional as sulcus haptic anterior CCC optic capture, ROC can be easily achieved by tucking each haptic of a sulcus-placed, one-piece IOL through the CCC.
1. Jones JJ, Oetting TA, Rogers GM, Jin GJ. Reverse optic capture of the single-piece acrylic intraocular lens in eyes with posterior capsule rupture. Ophthalmic Surg Lasers Imaging. 2012;43(6):480-488.
2. Gimbel HV, Amritanand A. Reverse optic capture to stabilize a toric intraocular lens. Case Rep Opthalmol. 2013;4(3):138-143.
3. Gimbel HV, Marzouk HA. Haptic tuck for reverse optic capture of a single-piece acrylic toric or other single-piece acrylic intraocular lenses. J Cataract Refract Surg. 2019;45:125-129.

John A. Hovanesian, MD
A Better Way to Achieve Scleral Fixation
I learned the Yamane technique for scleral fixation by sampling multiple videos on Eyetube and attending a course at the Kiawah Eye meeting, and I have since used it regularly and successfully. A video by Brandon D. Ayres, MD, has been particularly helpful (bit.ly/ayres1219H).
Editor’s note: Stay tuned for CRST’s February issue, which includes a spotlight on learning the Yamane technique.

Soosan Jacob, MS, FRCS, DNB
Human Tissue Ring Segments
I have devised a technique for keratoconus that I call corneal allogenic intrastromal ring segments (CAIRS; Figure 4).1 It solves the problems related to synthetic intrastromal corneal ring segments (ICRSs)—corneal melt, necrosis, infection, migration, extrusion, intrusion, and neovascularization—by using allogenic tissue for this purpose, and it retains the advantages of synthetic ICRS devices. In fact, I have found that CAIRS is better than implantation of synthetic ICRSs with respect to patients’ visual recovery and topographic improvement because they can be implanted more superficially. They can also be implanted in a wide range of disease from mild to severe. We have been able to avoid deep anterior lamellar keratoplasty in many patients by combining CAIRS with contact lens–assisted CXL. Synthetic ICRS devices such as Intacs (Addition Technology/Oasis Medical) and Keraring (Mediphacos; not available in the United States) have many advantages, but ophthalmologists are wary of these technologies due to the risks just enumerated (Figure 5). However, CAIRS takes care of these problems. We have used CAIRS to treat post-Intacs/Keraring corneal melts by replacing the synthetic segment with allogenic tissue.
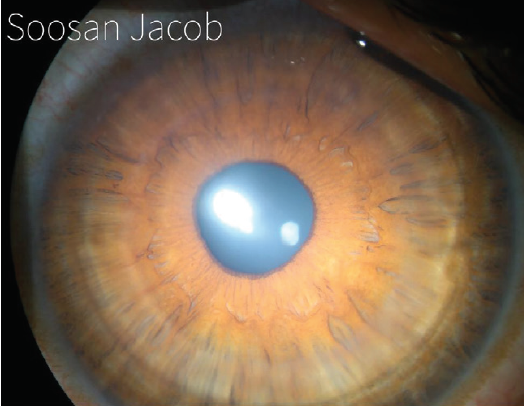
Figure 4. CAIRS implanted in the cornea of a keratoconic patient.
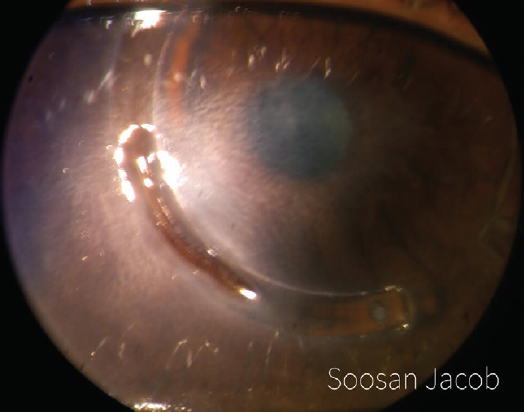
Figure 5. Synthetic ICRSs can be associated with a high risk of infections and other complications. This image shows Intacs-related corneal melt, necrosis, and segment exposure.
Courtesy of Soosan Jacob, MS, FRCS, DNB
Since publishing results of the first 24 cases in 2018,1 we have performed more than 120 procedures. CAIRS has now been performed by a few surgeons in other countries, including the United States. A video demonstration of CAIRS is viewable at bit.ly/jacob1219B.
1. Jacob S, Patel SR, Agarwal A, Ramalingam A, Saijimol AI, Raj JM. Corneal allogenic intrastromal ring segments (CAIRS) combined with corneal cross-linking for keratoconus. J Refract Surg. 2018;34(5):296-303.

Björn Johansson, MD, PhD, FEBO
You Don’t Always Need an NSAID
This year, I learned that the so-called overwhelming evidence that an NSAID should be used in every patient after cataract surgery may not necessarily be true. I learned this by checking whether my clinical impression matched the results from a systematic follow-up of 1,000 surgeries performed in our clinic in an unselected population.1 Because general use of NSAIDs did not appear to decrease the incidence of cystoid macular edema or the need for unplanned visits after cataract surgery, our clinic’s two units will be discussing optimal use of NSAIDs. I expect to implement a harmonized and optimized postoperative antiinflammatory protocol in the upcoming year.
1. Gärdin J, Johansson B. Unplanned visits after phacoemulsification with steroids only versus NSAID + steroids – a population-based comparative retrospective study. Paper presented at: the ASCRS/ASOA Annual Meeting; May 3-7, 2019; San Diego.

Aylin Kılıç, MD
Collecting Objective Data Before Surgical Planning
Cataract and refractive surgeons rely on patients to communicate their visual behaviors, and this subjective information is used by surgeons to devise a treatment plan, whether for cataract or refractive surgery. The surgical plan is an important decision for both surgeons and patients because it has permanent implications. In my experience, patients usually have difficulty describing their daily activities and what is important to them. A better approach to obtaining this information is by use of an objective visual behavior tool. The Vivior Behavior Monitor device (VBM; Vivior, Figure 6) combines data from the patient’s behavior with objective measurements.
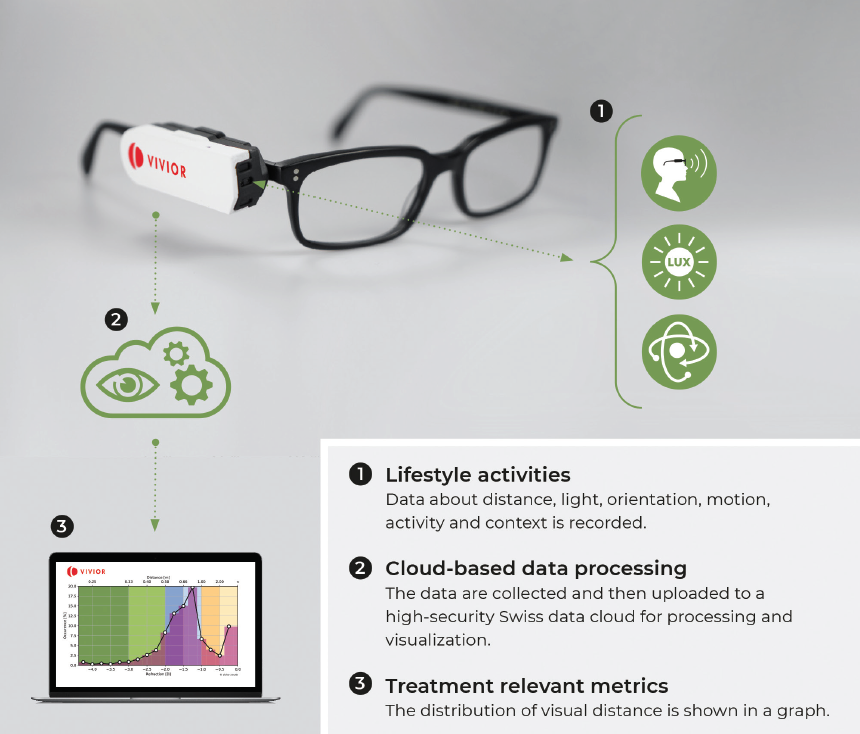
Figure 6. The VBM system attaches to the patient’s spectacles and monitors his or her visual behavior.
Courtesy of Aylin Kiliç, MD
Patients attach the VBM to their spectacles during a testing period—typically 8 to 10 hours a day for 3 to 5 days—so that the device can capture information about their individual viewing distances during daily activities, such as working on a computer or tablet, using a smartphone, gardening, and driving. The VBM provides intuitive reports for surgeons and also for patients; in particular, the patient report helps patients to understand the potential impact of vision correction surgery on their lifestyle. This can facilitate the patient’s conversation and decision-making process with the surgeon.
Another reason I like the technology is that it enables me to engage with my patients and show them that we care about them as individuals and can offer a treatment tailored to their needs.

William J. Lahners, MD, FACS
Personalized Vision
The best cataract pearl I picked up in 2019 was the personalized vision approach to implantation of presbyopia-correcting IOLs popularized by surgeons including Mark A. Kontos, MD; Steven J. Dell, MD; and Frank A. Bucci Jr, MD. This approach involves the use of a Symfony EDOF lens in the dominant eye and a Tecnis Multifocal lens (usually the model ZLB00 with +3.25 D add) in the other. This has been the most successful and most popular presbyopia-correcting surgery we have offered at our practice so far, with results surpassing those for bilateral implantation of either lens.

Cathleen M. McCabe, MD
Belt Loop Double Flange Technique for Scleral Fixation of a Dislocated IOL
This technique was developed for fixation of a dislocated IOL to the sclera using 5-0 polypropylene sutures. In brief, a piece of 5-0 polypropylene is cut, and the suture is placed through sclera, through the capsular bag, around the haptic, and out through the sclera. The ends are melted into a flange with low temperature handheld cautery in the same manner the haptics of a three-piece lens are fixated using the Yamane technique.1 Sergio Canabrava, MD, has described a similar double flange technique with 5-0 polypropylene to fixate a capsular tension ring (CTR) or segment.2
The belt loop technique is used for capsular bag and lens dislocations when the IOL will be fixated to the sclera without requiring an IOL exchange. The technique is described here, and video demonstrations can be seen at bit.ly/mccabe1219C, bit.ly/mccabe1219D, bit.ly/mccabe1219E, bit.ly/mccabe1219F, and bit.ly/mccabe1219G.
First, a 27-gauge needle is placed through conjunctiva and sclera 2 mm posterior to the limbus in the area of maximal zonular weakness. The needle is then passed through the capsular bag and under the haptic (and CTR if present). A cut piece of 5-0 polypropylene is threaded into the lumen of the 27-gauge needle. The polypropylene is cut at an angle to create a beveled end and facilitate the threading of the suture into the needle. The needle can be externalized through a paracentesis to make threading easier if desired. Alternatively, the suture can be threaded into the needle lumen in the anterior chamber with intraocular microforceps.
Once the 5-0 polypropylene is in the needle, the needle is carefully withdrawn from the sclera, grasped externally with forceps, trimmed, and melted with low temperature cautery to create a flange. A 27-gauge needle is then introduced through the conjunctiva and sclera 0.5 mm anterior to the original insertion, into the sulcus in front of the capsular bag and haptic, and into the anterior chamber. The opposite end of the 5-0 polypropylene is threaded into the needle, and the needle is then withdrawn from the sclera. The 5-0 polypropylene is slowly externalized to tighten the belt loop before it is trimmed and melted to create a second flange. The flanges are pushed back through the conjunctiva and into the sclera. A cannula is passed over the conjunctiva to confirm that the flange is flush with or within the sclera. If significant laxity is present on the opposite side of the capsular bag, a second belt loop can be placed 180º away to support the opposite haptic.
Advantages of this technique include the ability to retain the original dislocated IOL, to avoid an exchange, and to decrease the likelihood that an anterior vitrectomy will be necessary. This is especially desirable when the original IOL is an advanced technology lens. It also avoids vitreous loss and trauma to the endothelium or iris that can occur when an IOL or CTR is removed.
I have performed this technique on one-piece hydrophobic acrylic, three-piece, multifocal, and closed-loop acrylic IOLs, and it has worked equally well in all cases. A modification of the belt loop technique has been proposed by Thomas A. Oetting, MS, MD. In that version, which he shares on his Facebook page, the 5-0 polypropylene suture is kept on the needle and passed through the sclera and into the sulcus.
1. McCabe CM. Belt loop technique: a new method for scleral fixation of a dislocated IOL. Paper presented at: AECOS 2019 Summer Symposium; July 13, 2019; Deer Valley, Utah.
2. Yamane S, Sato S, Maruyama-Inoue M, Kadonosono K. Flanged intrascleral intraocular lens fixation with double-needle technique. Ophthalmology. 2017;124:1136-1142.
3. Canabrava S, Bernardino L, Batisteli T, Lopes G, Diniz-Filho A. Double-flanged-haptic and capsular tension ring or segment for sutureless fixation in zonular instability. Int Ophthalmol. 2018;38(6):2653-2662.

Beeran Meghpara, MD
Identifying and Treating Visually Significant Flap Striae
LASIK’s safety and efficacy are well documented in scientific research, FDA clinical trials, and National Eye Institute–sponsored studies. That said, no surgical procedure is 100% safe, and, although rare, complications can occur. One particularly frustrating complication of LASIK is flap striae. Centrally located flap striae can cause loss in visual acuity. This year, my top pearl involves identifying and treating visually significant striae as early as possible.
Large striae are more easily detectable because they can be seen with direct illumination; subtle striae, however, are best seen by using retroillumination though a dilated pupil (Figure 7) or by looking for negative staining after the instillation of fluorescein dye (Figure 8). Whether large or more subtle, any visually significant striae should be treated as soon as they are identified; they will not go away on their own.
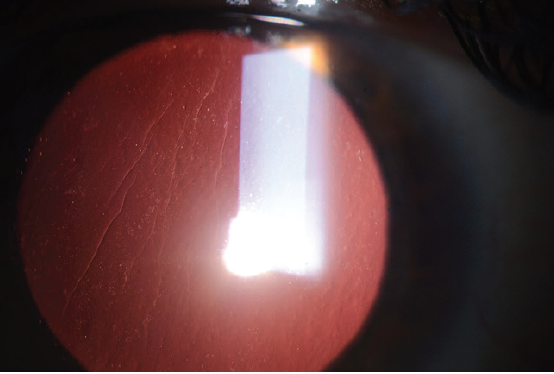
Figure 7. Flap striae viewed by retroillumination through a dilated pupil.
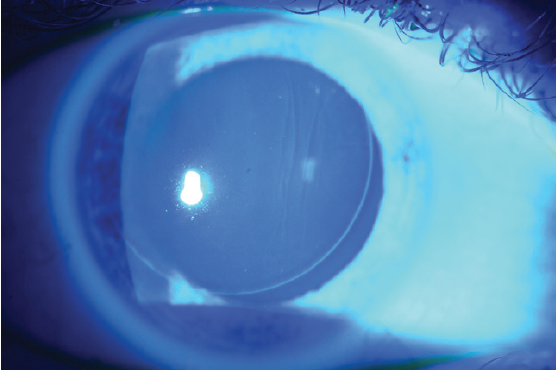
Figure 8. Negative staining of flap striae with fluorescein dye.
Early treatment is fairly straightforward and usually involves lifting the flap, stretching it with a cellulose sponge, and refloating it back into place. Striae that have been present for several weeks are much more difficult to treat, however. In these cases, simply lifting and replacing the flap is usually not effective because the corneal epithelium remodels and essentially acts as a tether to the striae. To treat folds of longer standing, a variety of techniques can be used, including removing the corneal epithelium over the striae, lifting the flap, swelling the flap with hypotonic saline, stretching it, and suturing it back down.
Suturing flap striae should be done with care. The sutures should be oriented perpendicular to the direction of the striae and must be tight enough to flatten the cornea but not so tight as to induce folds in the opposite meridian. In the event of severe striae, sutures may need to be placed along the entire flap edge. One trick I find helpful is to place a final suture though the hinge of the flap to balance suture tension; the final suture pattern resembles that of a corneal transplant (Figure 9). My preferred surgical technique is demonstrated in a video at this link: bit.ly/meghpara1219I.
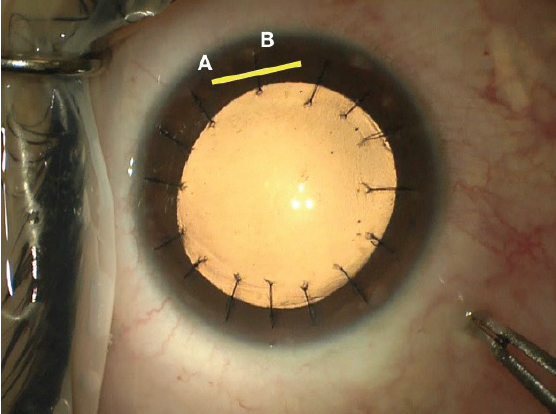
Figure 9. Intraoperative photograph following flap suturing. Note the location of the flap hinge marked with a yellow line (A) and the partial thickness suture placed through it (B).
Courtesy of Beeran Meghpara, MD

Michael Patterson, DO
Using Foveal Alignment to Your Benefit
Over the past decade, many advances have been made in the field of biometry. One of the best sources we now have for biometry is the IOLMaster 700 (Carl Zeiss Meditec). The precision of the device’s swept-source OCT measurements means that we are no longer unsure why interocular axial lengths are slightly different. We don’t have to guess if the eyes are truly different in length by 0.4 mm, a difference that can obviously alter IOL power calculations considerably. We know without question whether the technician lined the patient up properly.
Foveal registration sets this machine apart from other biometers and provides a much more exact IOL calculation. If the patient fixates just nasal or temporal to the fovea, this can completely derail a perfect cataract surgery outcome. Patients with dense cataracts can have a hard time finding the focusing beam on many biometers, but this machine eliminates the guesswork. Another benefit of swept-source OCT is that it can penetrate much denser lenses than previous models could. This has drastically reduced the number of A-scans our clinic has to obtain. While it hasn’t meant as much to me as the surgeon, because I don’t perform the A-scans, my staff is very pleased. And as they say, happy staff, happy life.

Kjell U. Sandvig, MD, PhD
Big Data and AI in Lens Calculations
What interested me most in 2019 is the potential that artificial intelligence (AI) presents in ophthalmology. I expect it to be a game-changer, not only for the detection and follow-up of diabetes, retinopathy, age-related macular degeneration, and glaucoma, but also for its use in IOL power calculations.
We have for many years achieved postoperative refractions within ±0.50 D of target in 86% of unselected cataract patients and within ±1.00 D in nearly 100%. These are fairly good results; however, we have not succeeded in further improving them, despite our use of new biometers and formulas. Now that AI-based formulas such as the Hill-RBF 2.0 (rbfcalculator.com) and the Pearl DGS (iolsolver.com) are available online, we probably have the tools we need for more precise calculations.
In data from about 12,500 eyes, use of the Hill-RBF formula resulted inpostoperative refractions within ±0.50 D of intended correction in 91% of 467 eyes.1 As this database increases with time, reaching true big data numbers, I expect even better results. The Hill-RBF formula is now integrated into the Lenstar 900 (Haag-Streit).
I am fascinated by the ability of powerful computers to see patterns in big data using multilayered neural networks that are modeled on our brains’ advanced networks, although those patterns are still not recognizable to our simple minds. I expect that deep machine learning, using hidden algorithms, will help us to predict outcomes better than ever.
1. Snyder ME. Hill-RBF calculator in clinical practice. CRST Europe. crstodayeurope.com/articles/new-frontiers-in-iol-prediction-for-improved-refractive-outcomes/hill-rbf-calculator-in-clinical-practice/. Accessed October 30, 2019.

Karl G. Stonecipher, MD
Planning for Topography-Guided Treatments
For me, the Phorcides vector calculation software program for topography-guided surgery with the WaveLight Refractive Suite (Alcon) has been a game-changer in 2019. The Phorcides software can outperform treatments calculated with manifest refraction or topography-modified refraction methods. In my experience, I have seen use of this technology lead to better postoperative UCVA in patients compared with planning based on manifest refraction or topography-modified refraction.

Mohamed Shafik Shaheen, MD, PhD
Sequential Wavefront-Guided PRK After CXL in Keratoconus
The top pearl I’ve used this year is performing sequential wavefront-guided PRK (WFG-PRK) in eyes with keratoconus that have achieved stability for at least 1 year after CXL. In order to maximize the accuracy of surgical planning, I measure the refraction a minimum of three times after CXL and check for agreement in the values.
My colleagues and I published a paper 3 years ago showing that, after 1 year of follow-up in patients with keratoconus and a previous history of CXL, WFG-PRK provided statistically significant improvements in uncorrected and corrected distance visual acuity corresponding to statistically significant reductions in mean manifest sphere and cylinder.1 Statistically significant improvements were also achieved in some topographic corneal irregularity indices and in total higher-order aberrations (HOAs), primary coma, and trefoil (Figure 10). There was no evidence of keratoconus progression after WFG-PRK.
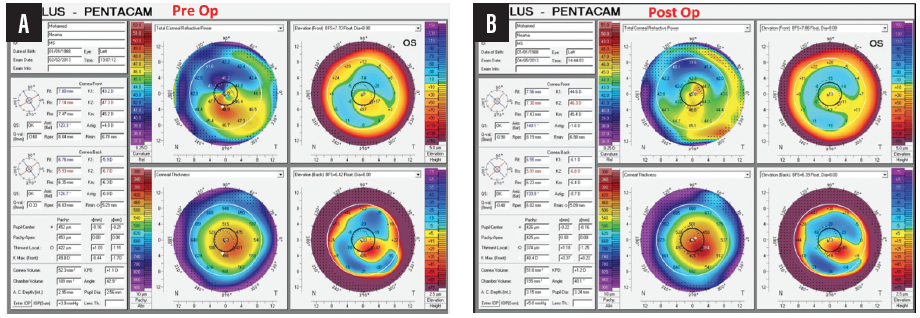
Figure 10. Pre- (A) and postoperative (B) topographies of an eye that underwent WFG-PRK after CXL for keratoconus.
Courtesy of Mohamed Shafik Shaheen, MD, PhD
I prefer a wavefront-guided ablation profile to a topography-guided one because the former addresses the HOAs that can degrade image quality in these complex corneas. Further, wavefront-guided platforms measure the central zone of the cornea, whereas topography-guided platforms extrapolate data to the central cornea. HOAs in the central cornea, primarily coma, are the main cause of vision degradation in eyes with keratoconus, and a high-definition aberrometer obtains exact measurements to calculate a proper and effective ablation profile.
Lastly, the planning software for WFG-PRK allows the surgeon to adjust the sphere and cylinder to correct the refractive error and target emmetropia. The advantages of wavefront-guided ablation to address irregular corneas were demonstrated in an earlier study.2
1. Shafik Shaheen MS, Shalaby Bardan A, Piñero DP, et al. Wave front-guided photorefractive keratectomy using a high-resolution aberrometer after corneal collagen cross-linking in keratoconus. Cornea. 2016;35(7):946-953.
2. Shafik Shaheen MS, El-Kateb M, Hafez TA, Piñero D, Khalifa MA. Wavefront-guided laser treatment using a high resolution aberrometer to measure irregular corneas: A pilot study. J Refract Surg. 2015;31(6):411-418.

William B. Trattler, MD
Improving Patients’ Ocular Surface Health
Manual expression of the meibomian glands, in conjunction with application of heat, can really make a difference for patients with meibomian gland dysfunction. This year, I have found it useful to use expression forceps developed by Sight Sciences after both TearCare (Sight Sciences) and LipiFlow Thermal Pulsation System (Johnson & Johnson Vision) heat treatments. Because these technologies are now quite affordable for patients, they can make a major impact on patients’ ocular surface health and can provide significant improvement in symptoms.




