CASE PRESENTATION
A 67-year-old man presents for cataract surgery. The patient has a history of bilateral monovision LASIK. The right and left eyes were targeted for distance and near vision, respectively. He does not have his old records. Nor does he remember his preoperative refraction, the name of the operating surgeon, or the laser that was used for the ablation. The patient is an avid golfer who plays weekly in all conditions and is proud of his 4 handicap.
Upon examination, the patient’s BCVA is 20/30-2 OD with a refraction of plano sphere and 20/30 OS with a refraction of -2.25 D sphere. His glare BCVA is 20/70 OD and 20/80 OS. A slit-lamp examination finds well-healed LASIK flaps, and there are no signs or symptoms of dry eye disease. Both eyes have an Ocular Surface Disease Index score of 8, no staining, a tear breakup time of 10 seconds, a grade 2+ to 3+ posterior subcapsular cataract, and 1+ cortical changes. A fundus examination of each eye is normal. The cup-to-disc ratio is 0.35 OU, and the IOP is 15 mm Hg OU. Measurements with the Pentacam (Oculus Optikgeräte) are shown in Figures 1 and 2.
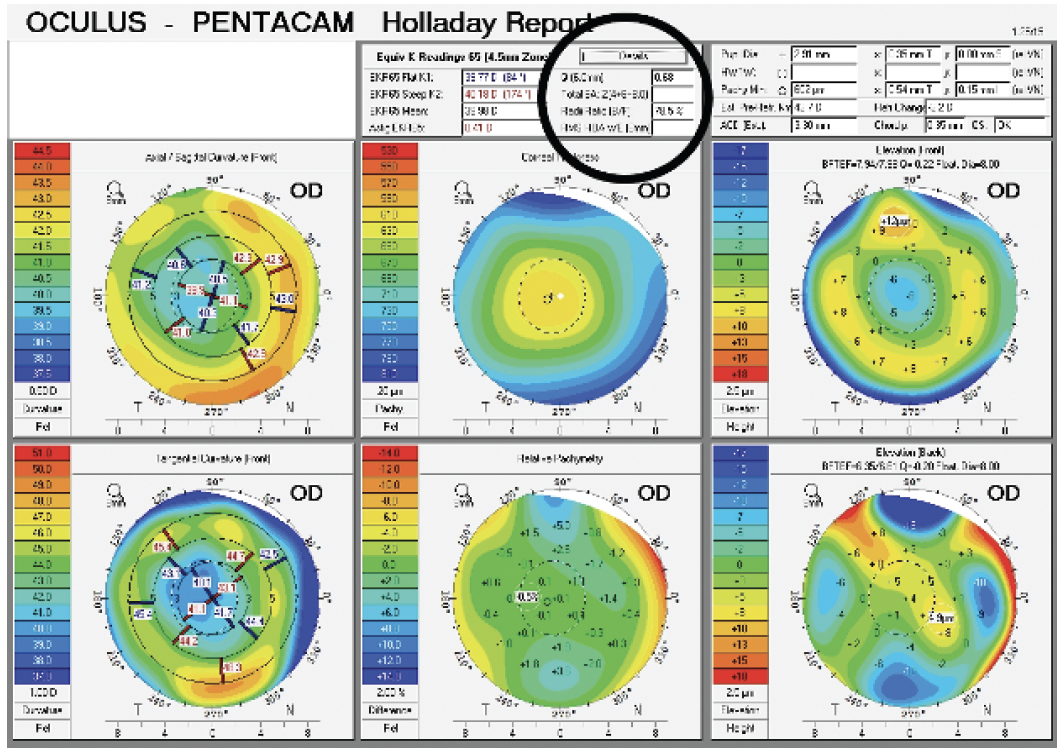
Figure 1. A typical myopic LASIK ablation with a Q value of +0.68.
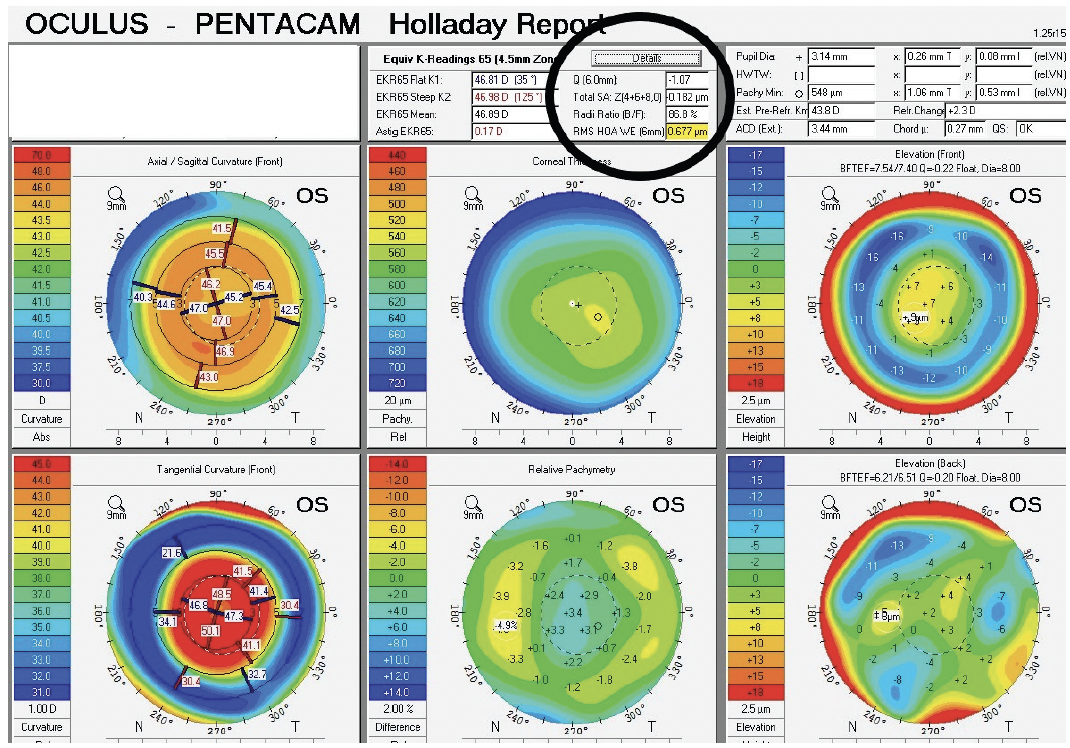
Figure 2. A typical hyperopic LASIK ablation with a Q value of -1.07.
The patient is willing to pay extra for surgery and advanced technology IOLs that have the potential to restore the vision he enjoyed shortly after LASIK.
How would you proceed?
—Case prepared by Karl G. Stonecipher, MD

ARTHUR B. CUMMINGS, MBCHB, FCS(SA), MMED(OPHTH), FRCSED, FWCRS
The average corneal Q value before any ocular surgery is -0.27. The value becomes more minus (prolate) after hyperopic LASIK and typically more positive (oblate) after myopic ablation. Corneal spherical aberration (SA) in an unoperated eye is around +0.20 to +0.27. Some spherical IOLs do not affect ocular SA (ie, the lens has the same SA as the eye’s natural lens SA). Aspheric IOLs such as the Tecnis (Johnson & Johnson Vision) reduce positive SA by 0.27 µm, and the AcrySof IOL (Alcon) reduces corneal SA by around 0.20 µm. An eye with 0.27 µm corneal SA that receives a Tecnis IOL with -0.27 µm of SA ends up with 0.00 µm of ocular SA and the highest quality of night driving vision. Implanting an AcrySof IOL in the same eye would reduce corneal SA to +0.07 µm in whole eye optics.
In this case, implanting a Tecnis IOL in the right eye would reduce the SA to +0.41 µm. Implanting the same IOL in the left eye increases the SA to -1.34 µm. The best quality of vision, especially for driving at night, requires ocular SA to be as close to zero as possible. Implanting a spherical IOL in the left eye would maintain -1.07 µm of ocular SA, which would provide depth of focus and is something to which the patient has historically adapted.
The fundamental question here is whether the patient was happy with his quality of vision before the cataracts developed. If so, I would place a spherical IOL (maintaining the +0.68 µm of SA) or an aspheric IOL in the right eye to reduce the positive SA and a spherical IOL in the left eye to maintain negative SA. If the patient was unhappy with his quality of vision, I would try to reduce the positive corneal SA in the right eye with a negative SA IOL.
It is important for surgeons to understand the difference between Q values, corneal SA, and whole eye SA. An eye with a high negative Q value has high positive corneal SA but negative whole eye SA. Such an eye has increased depth of focus even with an aspheric IOL.

DAMIEN GATINEL, MD, PHD
Because the patient is satisfied with monovision, it is appropriate to reproduce the strategy with cataract surgery and IOL implantation.
His history of corneal refractive surgery has important implications for biometry and IOL selection. Biometry would include measurements of the anterior chamber depth and crystalline lens thickness to increase the accuracy of the predicted effective lens position. The latest generation of IOL formulas would be used, ideally equipped with a specific module for eyes that have undergone refractive surgery (eg, PEARL-DGS with the complex eyes option) and an accurate estimate of total corneal power. The Pentacam, used in the case presentation, can estimate total corneal power with posterior surface data. The refractive targets would be emmetropia in the right eye and an amount of myopia in the left eye comparable to that which allowed the patient to read before the onset of cataracts.
I would select an IOL that induces negative SA in the right eye, promoting quality of vision by reducing the eye’s positive SA. An aberration-free IOL is indicated for the left eye. It seems inappropriate or even unwise to consider an extended depth of focus (EDOF) IOL in this situation because of the characteristics of the patient’s corneas and the unpredictable interactions between elevated corneal SA and the SA generated by these highly aspheric lenses. For example, if increased depth of focus is desired in the right eye, the IOL implanted should not cause negative SA (canceling out all or part of the corneal SA and resulting in little to no whole eye SA). An IOL that provides no or positive SA would be a better choice.

BEN LAHOOD, MBCHB, PGDIPOPHTH, PHD, FRANZCO, FWCRS
When a patient is happy with monovision before developing cataracts, I tend to replicate this strategy with monofocal IOLs. In my experience, if patients have a history of laser vision correction, EDOF and monofocal plus IOLs, both of which usually induce SA changes, are less predictable in terms of the refractive outcome. Many surgeons also avoid trifocal IOLs in this situation, but I find these lenses work well because patients tend to be highly motivated to achieve spectacle independence and accept visual side effects. IOL power calculations that use measured posterior cornea values and the Barrett True K formula for post–refractive surgery eyes tend to be the most accurate in these eyes.
The most noticeable difference between the patient’s two eyes is the shape of the corneas. As Dr. Cummings touched on, the average untreated human cornea has a prolate shape with a Q value of around -0.26 and SA of +0.27 µm. SA has the greatest impact on IOL style and design options because it can negatively affect quality of vision. Neutralizing SA with an aspheric monofocal IOL should optimize a patient’s visual acuity. Small amounts of positive SA in combination with myopia can enhance depth of focus. Emmetropia would be targeted in the right eye, and a monofocal IOL with negative SA would be implanted to reduce positive SA. The left eye has a hyperprolate shape. Moderate myopia would be targeted, and a spherical IOL would be implanted to reduce negative SA.
Before proceeding to surgery, I would explain to the patient that cataract surgery outcomes are less predictable in eyes that have undergone laser vision correction, meaning that he may require a postoperative enhancement. Any adjustment required postoperatively would be addressed with PRK given the time that has elapsed since LASIK. Replicating the monovision strategy while taking his SA into consideration should optimize his quality and range of vision.

IVAN MAC, MD, MBA
Cases such as this one will become more common as the earliest LASIK patients develop cataracts.
The current patient has tolerated monovision well. LASIK likely induced positive SA in the right eye and negative SA in the left. Multifocal and EDOF IOLs correct positive corneal SA, so they would worsen the negative SA in the left eye, probably leading to significant dysphotopsias. He has three main options.
Option No. 1: Full monovision would be maintained. An aspheric IOL would be implanted in the right eye with a target of plano. A spherical IOL would be implanted in the left eye with a target of -1.75 to -2.00 D. This approach should improve the higher-order aberrations in the right eye and not worsen them in the left. Based on my experience, the possible downside to this approach is that, as the patient ages, his ability to tolerate full monovision may decrease.
Option No. 2: A blended vision strategy with the Light Adjustable Lens (LAL; RxSight) would be pursued. The right and left eyes would be targeted for plano and -1.00 D, respectively. A plano target would typically improve the positive SA in the dominant right eye. Aiming for low myopia (-1.00 D of sphere) and maintaining the negative SA in the nondominant left eye could improve quality of vision for distance and depth of focus for near.
Option No. 3: An LAL would be implanted in the dominant right eye, and an IC-8 Apthera IOL (Bausch + Lomb) with a target of -0.75 D would be implanted in the left eye. The optics of the right eye would be improved with an LAL light adjustment to negate the positive SA. The Apthera’s 1.36-mm pinhole optic should significantly improve the patient’s quality of vision and depth of focus.

WHAT I DID: KARL G. STONECIPHER, MD
The retired patient is an avid golfer who wanted to improve his distance vision. Although he has friends and family members who have done well with trifocal IOLs, he expressed concern about possibly experiencing postoperative dysphtopsias and wanted to be able to play golf under all conditions. He desired the best optics and best optical quality from an IOL possible.
I recommended re-creating the monovision he had enjoyed after his original LASIK procedure, with the right eye targeted for distance and the left eye for -1.00 D. The potential loss of near vision was discussed. The patient decided to undergo surgery on the right eye first. I do not currently implant the LAL, but it would have been an option in this case.
Laser cataract surgery was performed on the right eye with guidance from intraoperative aberrometry using the ASCRS and ESCRS calculators. A Tecnis 1-Piece IOL (Johnson & Johnson Vision) was implanted. The refractive target was plano. The patient’s postoperative UCVA was 20/15 OD. He was highly satisfied but noted he could no longer view his cellphone when his left eye was closed.
Despite the significant cataract in the left eye, the patient was able to do a contact lens trial of various near vision options. He tolerated -0.75 D of defocus, which provided him with good depth perception and enough functional near vision without glasses for most of his activities. We discussed the potential benefits of implanting a RayOne EMV lens (Rayner) in his left eye. My thought was that the IOL’s positive SA would reduce the eye’s Q value and a -0.50 to -0.75 D offset target would provide him with intermediate visual acuity. The patient agreed to this plan.
Laser cataract surgery was performed on the left eye with guidance from intraoperative aberrometry using the ASCRS and ESCRS calculators, and a RayOne EMV lens was implanted. Postoperatively, the patient’s refraction was -0.50 D sphere OS, and his UCVA was 20/25 OS.
The patient was satisfied with his distance and intermediate vision. He was able to function without spectacles for approximately 90% of his day and wore reading glasses when necessary.




