

The corneal endothelium is composed of a single layer of corneal endothelial cells (CECs). These cells exhibit limited proliferation, largely owing to contact inhibition and the presence of transforming growth factor beta in the aqueous humor. As a result, healing of the corneal endothelium primarily occurs through cell migration and enlargement rather than cell division. Various forms of treatment leverage these healing characteristics. For example, during Descemet stripping only for mild Fuchs endothelial corneal dystrophy,1,2 Descemet membrane is removed from the central cornea to encourage healing in the peripheral corneal endothelium.
Corneal endothelial dysfunction arises from impaired cellular pump and barrier functions. It is characterized by extensive guttae formation or substantially reduced CEC density. Dysfunction can occur in eyes with various conditions, including moderate to advanced Fuchs endothelial corneal dystrophy, pseudoexfoliation syndrome, and viral corneal endotheliitis. It can also be a consequence of endothelial trauma during surgery (eg, cataract, glaucoma, vitreoretinal, laser iridotomy) and chronic corneal graft failure. Corneal endothelial transplantation techniques employ donor grafts to restore corneal transparency.3
Treatments for limbal stem cell deficiency depend on the ongoing proliferation of corneal epithelial progenitor/stem cells. Corneal endothelial transplantation, in contrast, does not require postoperative cell growth. Consequently, surgical approaches to corneal endothelial failure are distinct from those addressing limbal deficiency.
Concept and Strategy
Two primary cell-based approaches are thought to restore the corneal endothelium. The first involves transplanting cultured CECs onto damaged corneal tissue. Success requires the transplanted cells to release beneficial molecules that help regenerate and reorganize the host cells, thereby restoring corneal function.
The second strategy, which is central to our innovative therapy, focuses on actual endothelial replacement. Surgically transplanted CECs replace the damaged or diseased endothelial layer. This form of therapy is grounded in the concept of true endothelial cell replacement rather than just regeneration of the host cells.
Research and Development
Researchers have extensively investigated the cultivation of human CECs, the in vitro proliferation of which is notoriously difficult. Recent advances in cellular biology are addressing the challenge. Cells from healthy corneal endothelium cultured in vitro exhibit heterogeneity in their structure and function (Figure 1). To increase surgical success and improve postoperative CEC density, we have found it essential to cultivate mature CECs with high purity and differentiated cells that closely resemble healthy in vivo CECs (Figure 2). More than expressing Na+-K+-ATPase and ZO-1 is required; the quality of control of cultured CECs must also take cell density, various cell surface markers, and other parameters into account.4,5
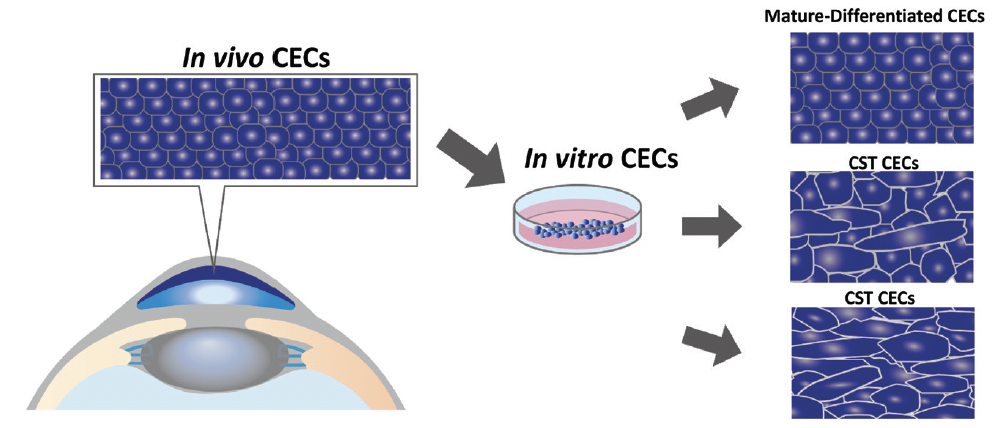
Figure 1. A schematic representation of cultured CECs.
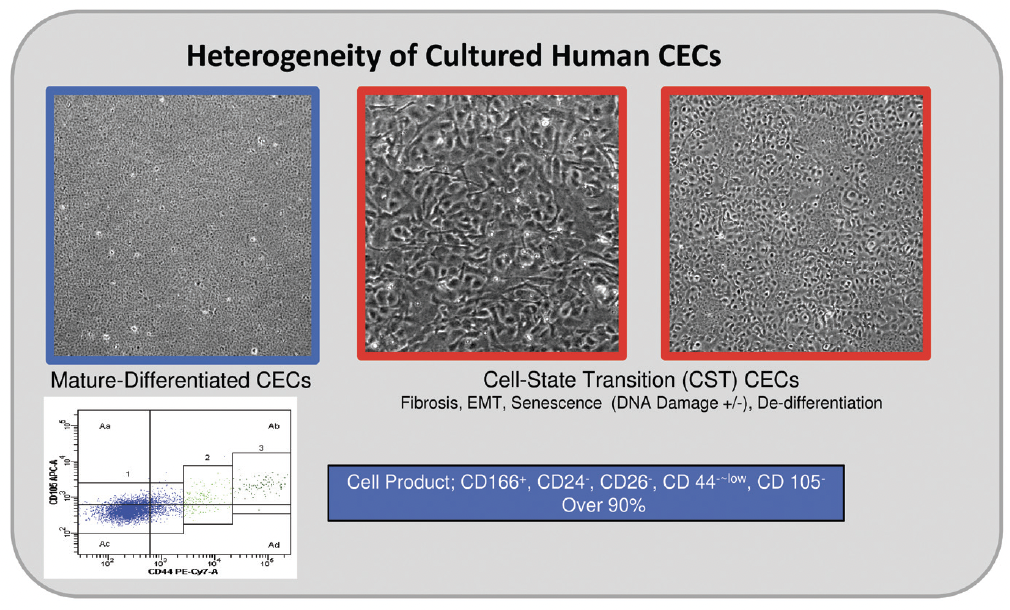
Figure 2. Highly mature-differentiated cultured CECs (highlighted in blue) resembling healthy in vivo CECs are crucial for effective transplantation. These CECs express the unique cell surface markers.
Safety and Quality Assurance
For cultured CECs to be clinically viable, they must undergo extensive testing for bacteria, viruses, and endotoxins to confirm the absence of mycoplasma contamination and ensure the sterility of the culture medium. Because many cells from a single donor cornea are distributed to multiple patients in one batch, strict adherence to safety guidelines is imperative to prevent adverse events.
Mature, differentiated CECs are nontumorigenic and exhibit no chromosomal abnormalities. Young donor corneas are chosen for in vitro culture, and they are processed at a cell processing center according to standard operating procedures that comply with Good Manufacturing Practice guidelines. Cell lots for clinical use undergo a comprehensive examination to ensure they meet rigorous clinical standards. Ultimately, a sterile suspension of CECs is prepared for transplantation therapy.6
Surgical Procedure
A small incision is made at the corneal limbus under local anesthesia. A silicone-tipped cannula is used to carefully remove abnormal extracellular matrix from Descemet membrane and degenerated CECs from the posterior surface of the cornea. A prepared suspension of cultured CECs with a Rho kinase inhibitor is injected into the anterior chamber with a 26-gauge needle attached to a dead-space free syringe.7,8
Postoperatively, patients lie face down for 3 hours to enhance cell adhesion to the targeted area. Topical steroids and antimicrobial agents are administered, following standard corneal transplantation protocols.
Clinical Results
The initial clinical trial, including 5 years of postoperative follow-up, found CEC transplantation therapy to be safe and effective for fully restoring the cornea in patients with corneal endothelial failure. Normal corneal thickness was restored, and complete resolution of corneal edema was observed and sustained for 5 years.8 At 9 years, favorable outcomes were consistently observed (Figure 3). The CECs, developed through the first-generation culture protocol, effectively repopulated on Descemet membrane and the corneal stroma’s bare posterior surface, demonstrating their biologic functionality and longevity.
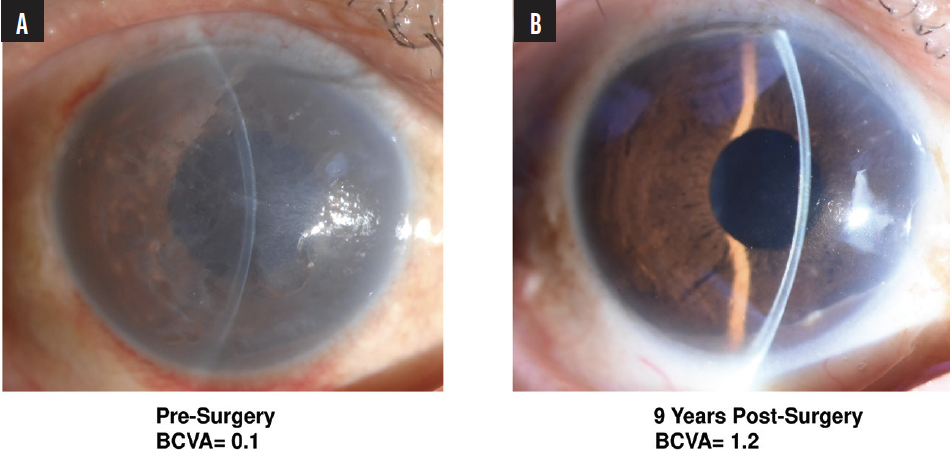
Figure 3. An eye before (A) and 9 years after (right) undergoing cultured CEC transplantation therapy for corneal endothelial failure.
Our study showed similar or slightly superior outcomes with regard to CEC density, corneal graft survival, and postoperative visual acuity compared to 5-year postoperative data on Descemet stripping automated endothelial keratoplasty and Descemet membrane endothelial keratoplasty.8,9 The use of second-generation CECs enhanced corneal restoration, both in terms of CEC density and recovery speed, likely due to rapid functional recovery from corneal dehydration. Three years postoperatively, the CEC density in eyes treated with second-generation cultured human CECs remained high with minimal decline, indicating long-term stability and rejuvenation of the CEC layer.10,11
There were no cases of immunologic rejection, uveitis, infection, or increased IOP directly related to the therapy. Phase 2 and 3 clinical trials in Japan involving 65 eyes and replication studies in El Salvador involving approximately 90 eyes also yielded favorable outcomes (unpublished data). Vyznova (Aurion Biotech) was subsequently approved by the Japanese Ministry of Health, Labour and Welfare in March 2023.
Conclusion
Further research is required to elucidate the relationship between CEC quality, corneal endothelial disease, and the anterior chamber environment and refine CEC transplantation therapy indications.12 Interest in methods for large-scale cell expansion, simplified cell delivery, and optimized cryopreservation grows as the understanding of CEC biology expands. Cultured CECs are poised to become the global standard for treating severe corneal endothelial disorders. The ability of injected CECs to organize themselves and rebuild the endothelial layer allows significant advancement of medical science and clinical applications. The early postoperative use of a topical Rho kinase inhibitor may enhance outcomes.
1. Borkar DS, Veldman P, Colby KA. Treatment of Fuchs endothelial dystrophy by Descemet stripping without endothelial keratoplasty. Cornea. 2016;35(10):1267-1273.
2. Moloney G, Garcerant Congote D, Hirnschall N, et al. Descemet stripping only supplemented with topical ripasudil for Fuchs endothelial dystrophy: 12-month outcomes of the Sydney Eye Hospital Study. Cornea. 2021;40(3):320-326.
3. Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379(9827):1749-1761.
4. Toda M, Ueno M, Hiraga A, et al. Production of homogeneous cultured human corneal endothelial cells indispensable for innovative cell therapy. Invest Ophthalmol Vis Sci. 2017;58(4):2011-2020.
5. Hamuro J, Deguchi H, Fujita T, et al. Polarized expression of ion channels and solute carrier family transporters on heterogeneous cultured human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2020;61(5):47.
6. Kinoshita S, Ueno M. Cultivated cells in the treatment of corneal diseases. In: Colby K, Dana R, eds. Foundations of Corneal Disease. Springer; 2020:215-224.
7. Kinoshita S, Koizumi N, Ueno M, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N Engl J Med. 2018;378(11):995-1003.
8. Numa K, Imai K, Ueno M, et al. Five-year follow-up of first 11 patients undergoing injection of cultured corneal endothelial cells for corneal endothelial failure. Ophthalmology. 2021;128(4):504-514.
9. Deng SX, Lee WB, Hammersmith KM, et al. Descemet membrane endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2018;125(2):295-310.
10. Ueno M, Toda M, Numa K, et al. Superiority of mature differentiated cultured human corneal endothelial cell injection therapy for corneal endothelial failure. Am J Ophthalmol. 2022;237:267-277.
11. Yamamoto A, Tanaka H, Toda M, et al. A physical biomarker of the quality of cultured corneal endothelial cells and of the long-term prognosis of corneal restoration in patients. Nat Biomed Eng. 2019;3(12):953-960.
12. Yamaguchi T, Higa K, Suzuki T, et al. Elevated cytokine levels in the aqueous humor of eyes with bullous keratopathy and low endothelial cell density. Invest Ophthalmol Vis Sci. 2016;57(14):5954-5962.




