CASE PRESENTATION
A 20-year-old man is urgently referred for evaluation of a cataract in his right eye by a local retina specialist. Several years ago, the patient, a college student, underwent a retinal detachment (RD) repair with silicone oil in the eye after sustaining trauma during a soccer game. The oil was removed 1 month ago. He experienced a rapid decline in quality of vision shortly thereafter.
The patient says his right eye could see reasonably well with a contact lens until just a few days after the silicone oil was removed. Aware that a cataract is forming in the right eye, the patient and his family want surgery to be performed before he returns to school in 2 weeks. He typically wears a -10.00 D soft contact lens on each eye.
On examination, the patient’s BCVA is 20/200 OD and 20/20 OS. IOP readings with Goldmann applanation tonometry are 19 mm Hg OD and 12 mm Hg OS. A slit-lamp examination of the right eye finds a clear cornea with peripheral scars from prior surgery. The chamber is deep with rare cell. Milky white changes to the crystalline lens and a 3+ anterior subcapsular cataract are evident. Cortical material is observed to be escaping into the anterior chamber from under the iris in the superior quadrant (Figure 1). The posterior segment is difficult to visualize, but B-scan ultrasound of the right eye is normal. Topography and biometry are shown in Figures 2 to 4. The left eye’s appearance is essentially normal at the slit lamp.
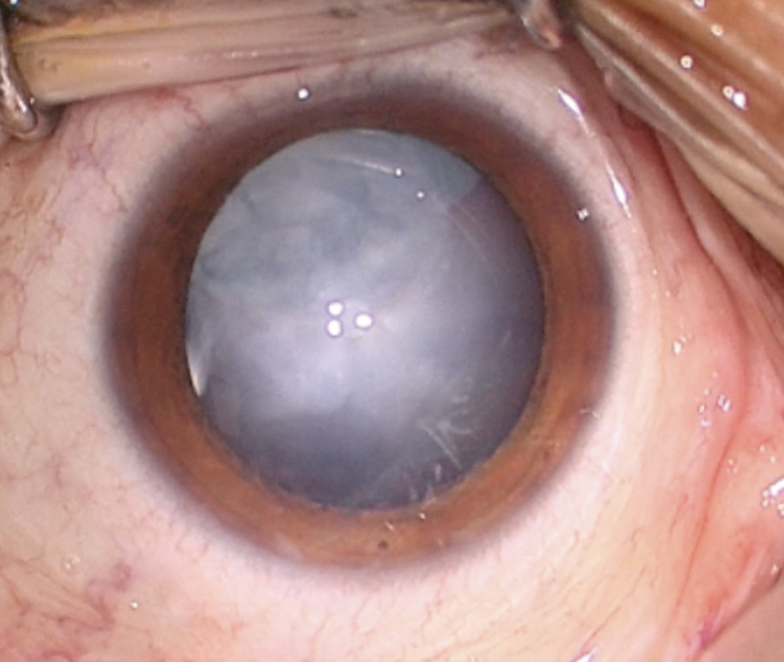
Figure 1. Anterior segment examination of the right eye. Note the milky white nuclear and anterior subcapsular changes. Although difficult to see here, a small amount of cortical material and small silicone oil bubbles are visible in the anterior chamber.
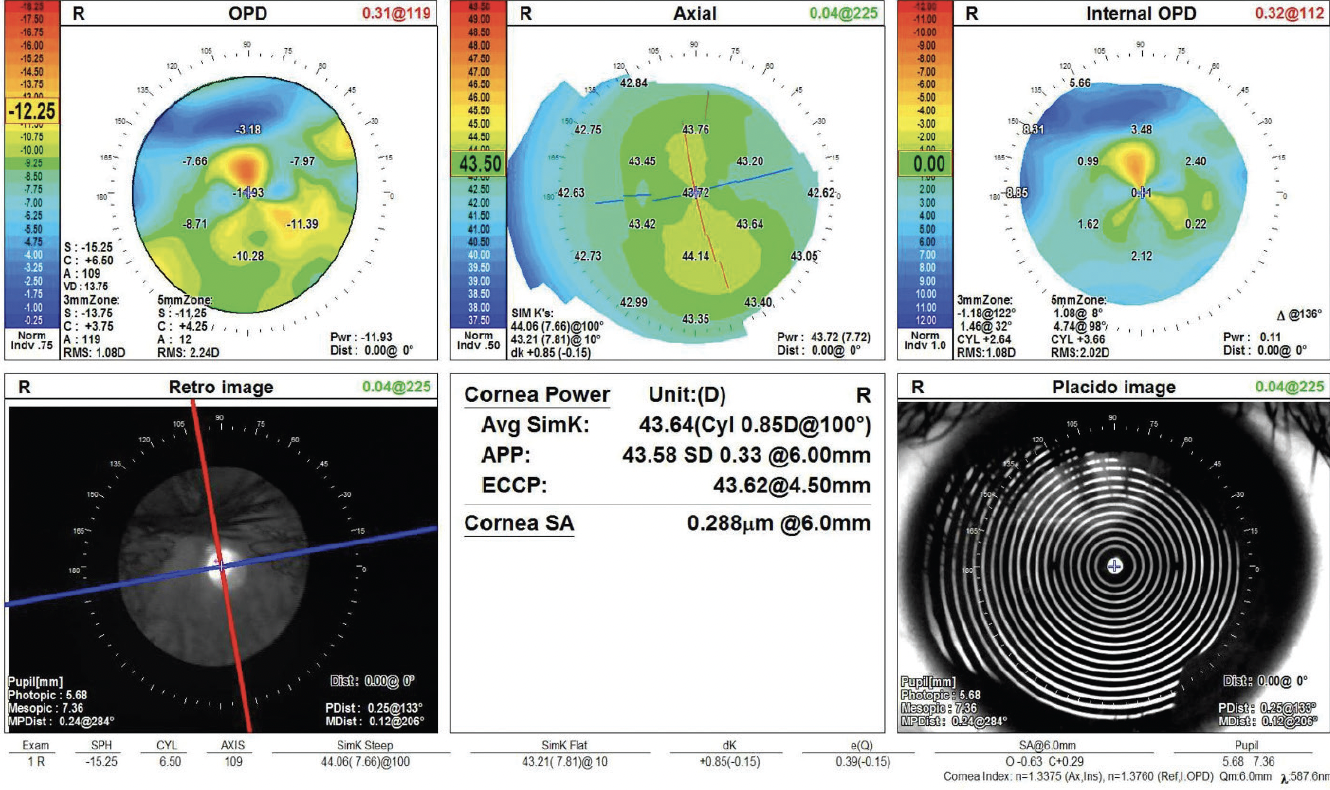
Figure 2. Topography of the right eye shows a healthy ocular surface and with-the-rule astigmatism.
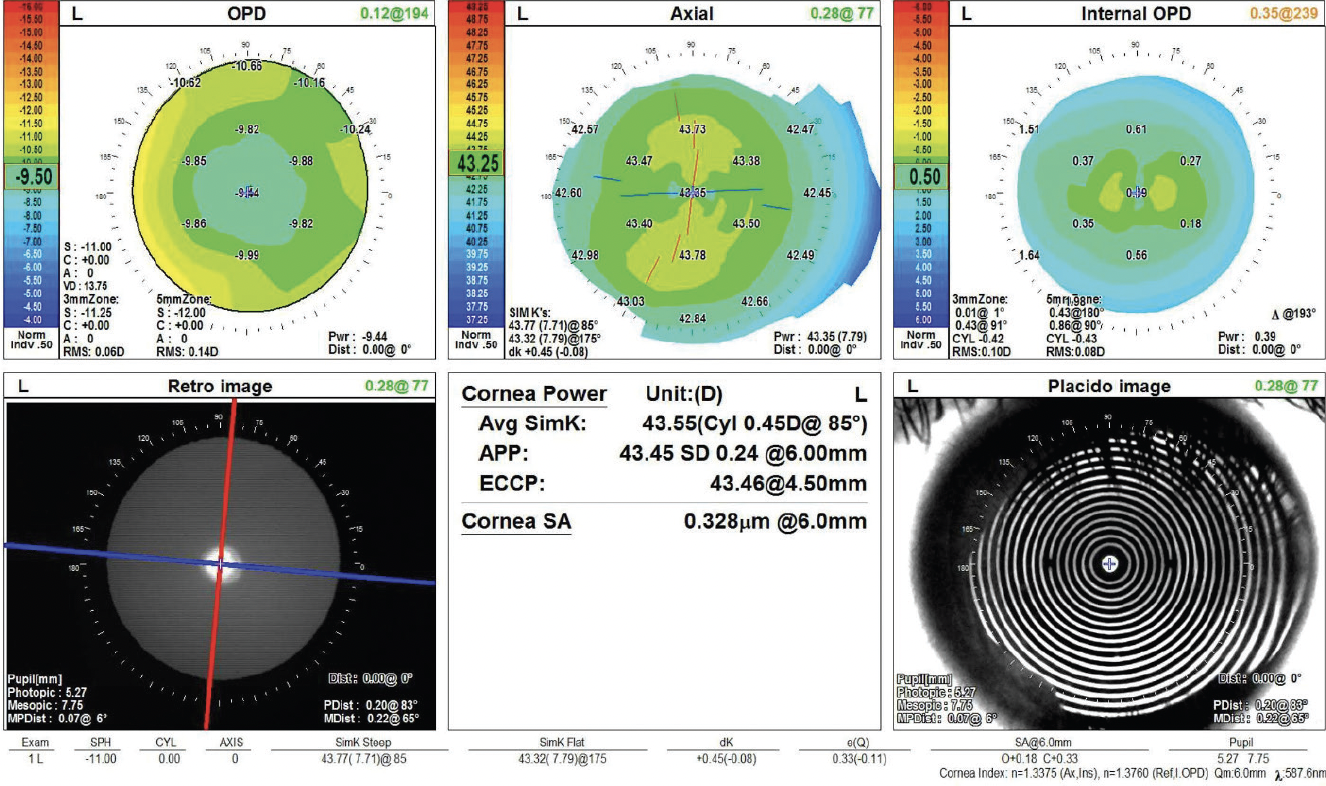
Figure 3. Topography of the unaffected left eye.
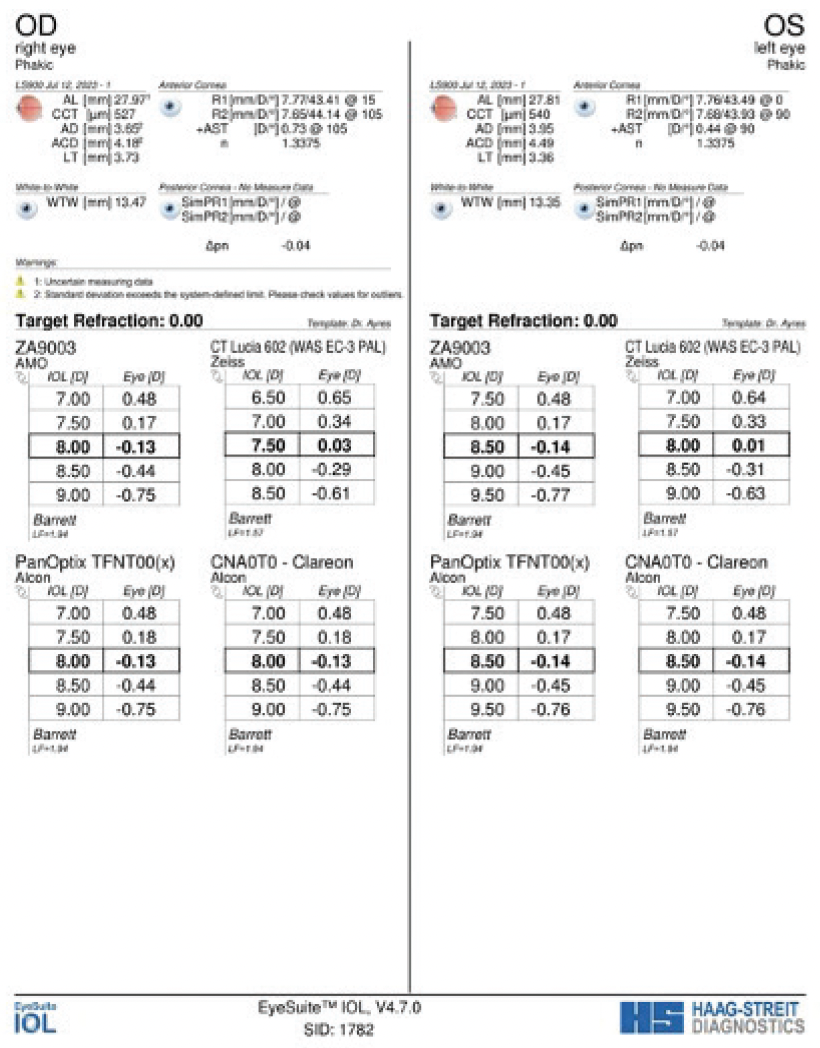
Figure 4. Biometry measurements of both eyes.
How would you manage the cataract in the right eye and the refractive error in the left eye to prevent anisometropia.
— Case prepared by Brandon D. Ayres, MD

NICHOLAS HADJOKAS, MD
The risks of surgery would be discussed with the patient and his family. Delaying surgery may allow the capsule to scar, but the level of risk would still be higher than for routine cataract surgery. If a decision is made to proceed at this time, coordination with a retina specialist would be considered owing to potential posterior dislocation of lens material.
The anterior capsule would be painted with trypan blue dye to facilitate assessment of the capsular rent. If the tear is peripheral and posterior, a continuous curvilinear capsulorhexis and gentle viscodissection of the lens would be performed. The lens would then be extracted with minimal rotation, and cortex would be removed with bimanual irrigation and aspiration. The eye would be refilled with an OVD before the irrigation handpiece is withdrawn from the eye. Depending on the size of the tear and the integrity of the capsulorhexis, either a one-piece IOL would be placed in the bag, or a three-piece IOL would be implanted in the sulcus with reverse optic capture.
Before cataract surgery, I would discuss with the patient his visual goals and explain that the eye’s ability to accommodate would be lost after surgery. Biometry suggests he would do well with a monofocal IOL. Given the patient’s long-standing myopia, he may wish to preserve his near visual acuity and continue wearing contact lenses in both eyes for distance. Regardless of his visual goals, he will require a contact lens in the left eye postoperatively. If he desires more permanent vision correction and the eye exhibits refractive stability, PRK or LASIK could be an option in the future.

JOSHUA C. TEICHMAN, MD, MPH, FRCSC
Capsular breaches can heal over time. Delaying surgery, however, is not an ideal option here, given that lens material is actively leaking, and the patient and his family would like surgery to be performed before he returns to school.
The patient’s use of contact lenses simplifies refractive planning somewhat. One possible approach would be to target a plano refraction in the right eye and inform the patient that he will experience absolute presbyopia and require spectacles or a contact lens for all near and intermediate tasks postoperatively, although the vision in his left eye may allow him to function without correction. To manage the anisometropia, he could continue wearing a contact lens on the left eye or undergo refractive surgery, either laser vision correction or phakic IOL (EVO ICL, STAAR Surgical) implantation depending on his candidacy. I would avoid refractive lens exchange in the left eye given the risk of RD.
An alternative approach would be to target the right eye for intermediate or near vision and inform the patient that he will need glasses or a contact lens for distance tasks and will experience monovision without correction of the right eye. Unfortunately, if he has no history of monovision, a contact lens trial is not currently an option. Anecdotally, however, I have found that athletes are typically more troubled by the loss of high-quality stereopsis than nonathletes.
Multifocal IOL implantation would be a third option. The loss of contrast sensitivity and potential postoperative dysphotopsias, however, may be particularly bothersome to this young, healthy, athletic patient. I would therefore be disinclined to pursue this avenue. The choices of IOL and refractive target, however, are ultimately his to make after informed consent.
During surgery, it would be important to avoid overfilling or overpressurizing the anterior chamber or allowing it to become shallow. Fluctuations in IOP and anterior chamber depth could allow the preexisting tear in the posterior capsule to extend and the entire lens to fall posteriorly. Balanced salt solution or an OVD would therefore be injected through the paracentesis whenever instruments are being removed from the main incision.
The anterior capsule would be stained with trypan blue dye. The capsule would then be punctured with a 25-gauge needle on a 3-mL syringe half filled with balanced salt solution. This should facilitate a controlled entry that does not apply posterior pressure. The capsulorhexis would be sized slightly smaller than my usual to allow optic capture of a sulcus-fixated IOL, if needed. Given the patient’s age, it may be possible to remove the crystalline lens with irrigation and aspiration alone (ie, no phacoemulsification). A low IOP would be maintained intraoperatively. Because the eye has been vitrectomized, vitreous prolapse is unlikely. Nevertheless, should vitreous remain in the eye, I would be careful to avoid traction, which poses an RD risk, and to avoid pulling vitreous anteriorly, which could enlarge the capsular defect. All silicone oil droplets would be removed to prevent endothelial decompensation.
A posterior capsular defect that is not round can be torn with microinstruments to create a round or complete posterior capsulorhexis. This maneuver, however, can be challenging in an eye without good support (ie, already vitrectomized). Inserting an IOL into the bag when the posterior capsule has torn risks splitting the capsule. If the capsule is deemed safe for IOL implantation intraoperatively, a one-piece toric monofocal IOL would be a reasonable choice. If the capsular defect is cause for concern, a three-piece IOL would be implanted in the sulcus, with the optic captured in the anterior capsulorhexis. Postoperatively, limbal relaxing incisions could be made at the slit lamp to address residual astigmatism.
A CT Lucia 602 IOL (Carl Zeiss Meditec) would be available in the OR. If both the anterior and posterior capsules are compromised, intrascleral haptic fixation of this lens with the Yamane technique would be performed.

WHAT I DID: BRANDON D. AYRES, MD
A detailed discussion of the risks and benefits of cataract surgery was held with the patient and his family. The potential complications and challenges of an open anterior capsule were explained, as was the possibility of additional surgery to remove the cataract completely if violation of the posterior capsule were detected. Toric and multifocal IOL options were discussed. Given the retinal pathology and condition of the anterior capsule, however, a monofocal IOL and target near plano were chosen. The plan was for the patient to continue wearing a contact lens on the left eye postoperatively to manage the surgically induced anisometropia and consider LASIK after finishing school.
The changes to the crystalline lens were found to have advanced significantly when he arrived in the OR 1 week later. Under topical anesthesia, two small paracentesis incisions were created at approximately the 11 and 7 clock positions. Preservative-free lidocaine was administered. A small amount of an OVD was instilled to form the anterior chamber. The cortical material that had prolapsed into the anterior chamber and blocked the view of the anterior capsule was removed with bimanual irrigation and aspiration (Figure 5).
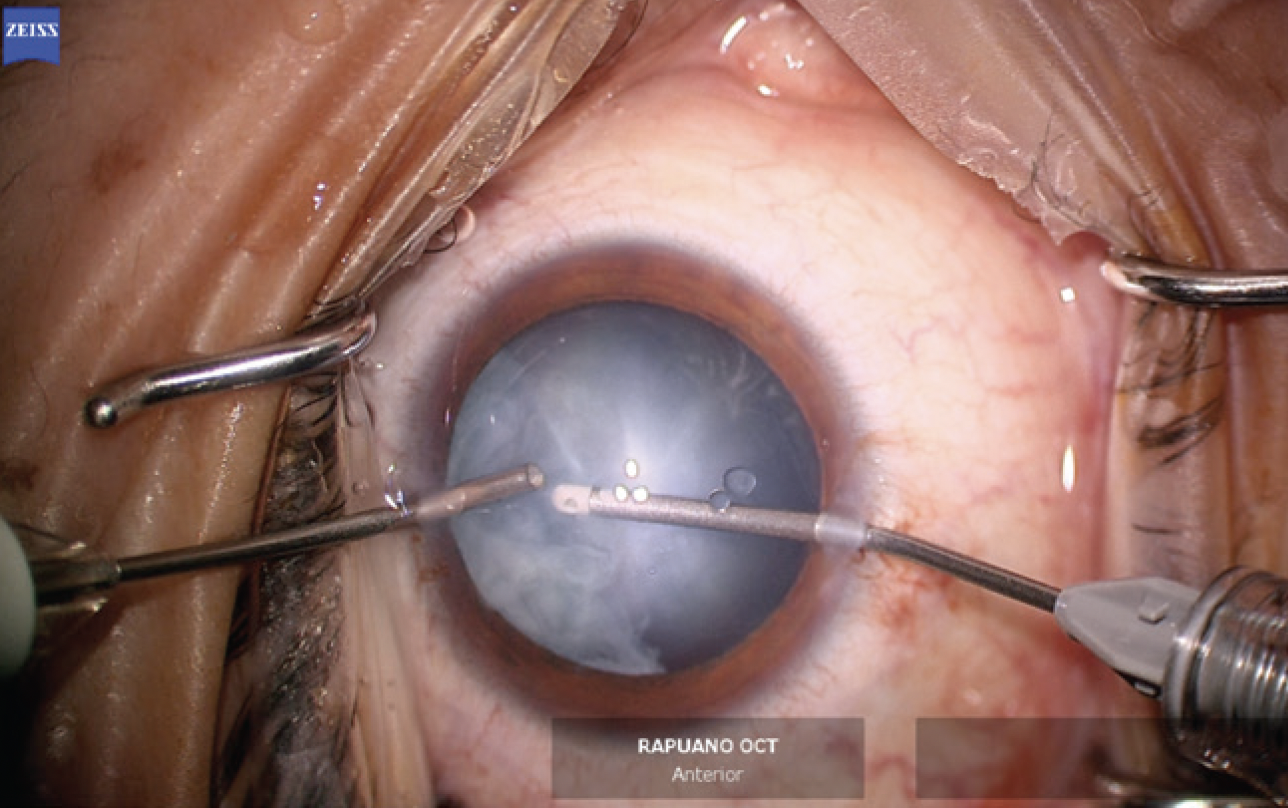
Figure 5. Bimanual irrigation and aspiration are performed to clear cortical lens material that has prolapsed into the anterior chamber and improve visualization of the anterior capsule.
The anterior chamber was re-formed with OVD, and a temporal 2.4-mm incision was created. The anterior capsule was painted with trypan blue dye to highlight the extent of the capsular tear. The capsulorhexis was initiated in the area of the violation with microscissors (Figures 6 and 7). A hemi-rhexis was created with microcapsulorhexis Utrata forceps. The remaining lens material was removed with bimanual irrigation and aspiration (Figure 8). Each time the irrigation unit was withdrawn from the eye, an additional amount of OVD was instilled to prevent shallowing of the anterior chamber and posterior extension of the capsular defect.
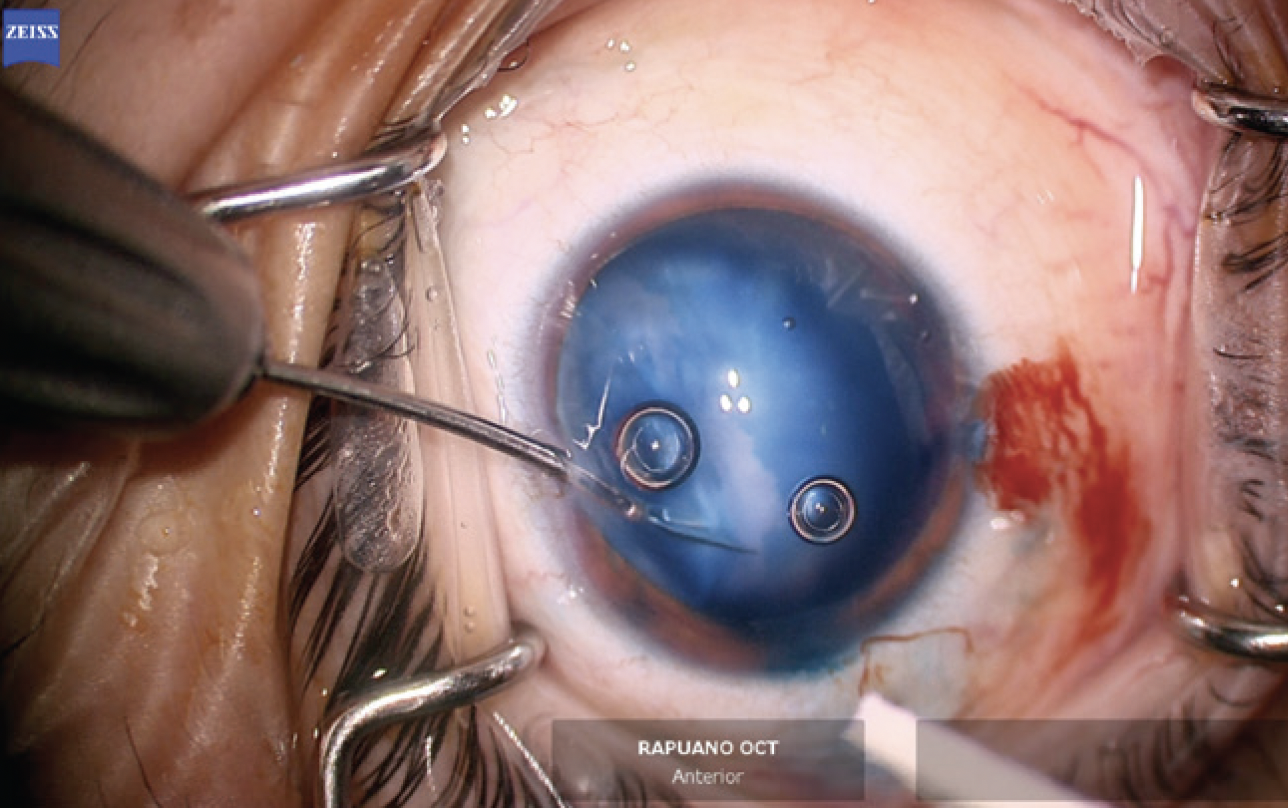
Figure 6. The capsulorhexis is initiated with microscissors at the edge of the capsular violation. Staining with trypan blue dye improves visualization of the anterior capsule.
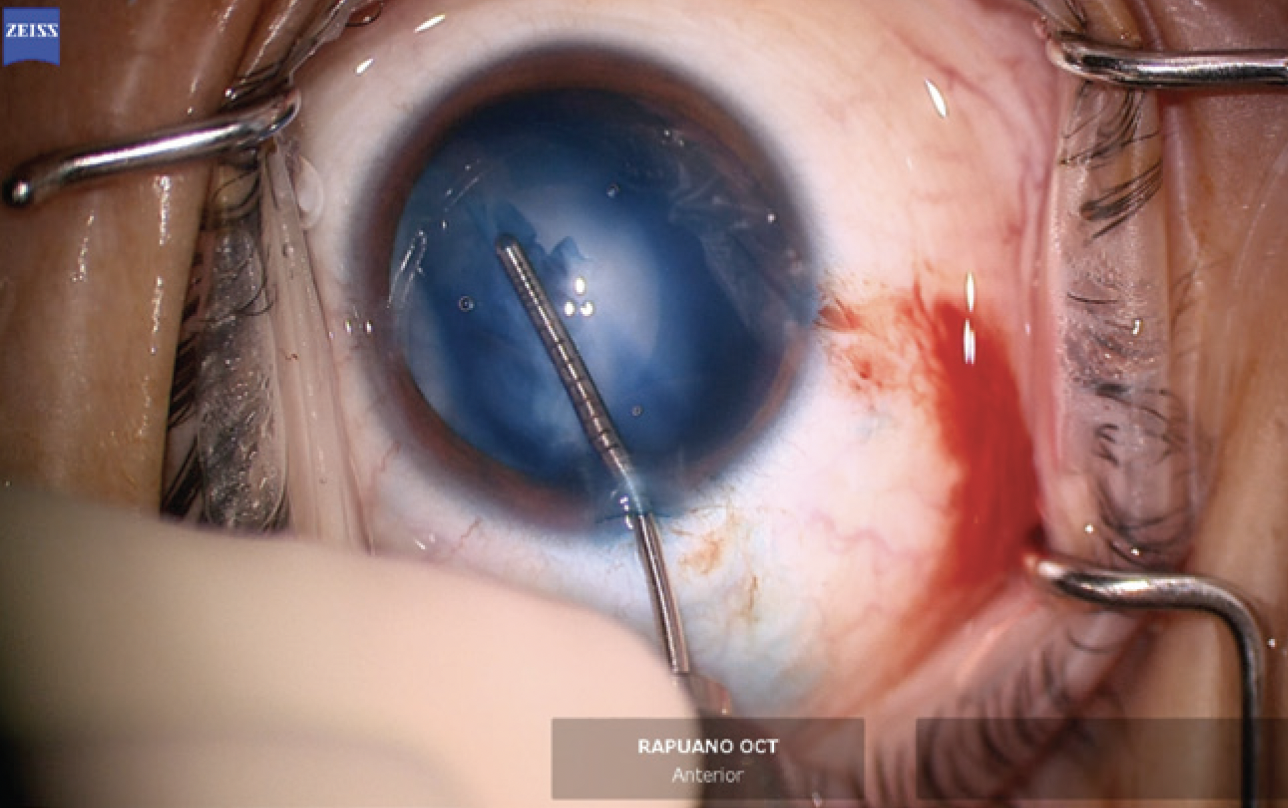
Figure 7. A hemi-rhexis performed with microcapsulorhexis forceps starts and ends at the edge of the preexisting capsular defect.
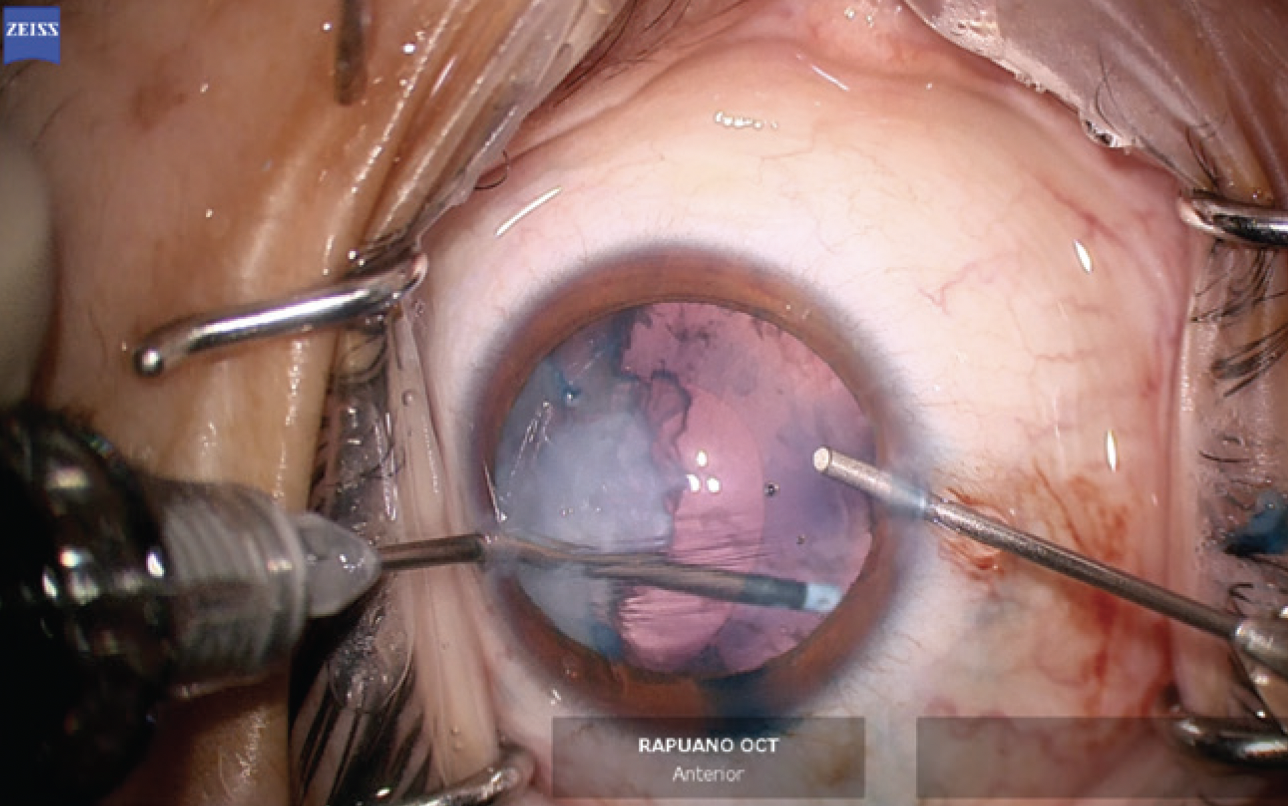
Figure 8. Lens material is removed with bimanual irrigation and aspiration.
The anterior hemi-rhexis was enlarged with microscissors and Utrata forceps (Figure 9) to prevent excessive capsular overlap of the IOL optic. A one-piece acrylic IOL was then placed in the capsular bag. The haptics were oriented to the 3 and 9 clock positions to ensure they were fully in the bag and prevent chafing against the iris and potential inflammation (Figure 10). The OVD was thoroughly removed from the eye, and the incisions were hydrated to prevent wound leak. An intracameral injection of moxifloxacin was performed.
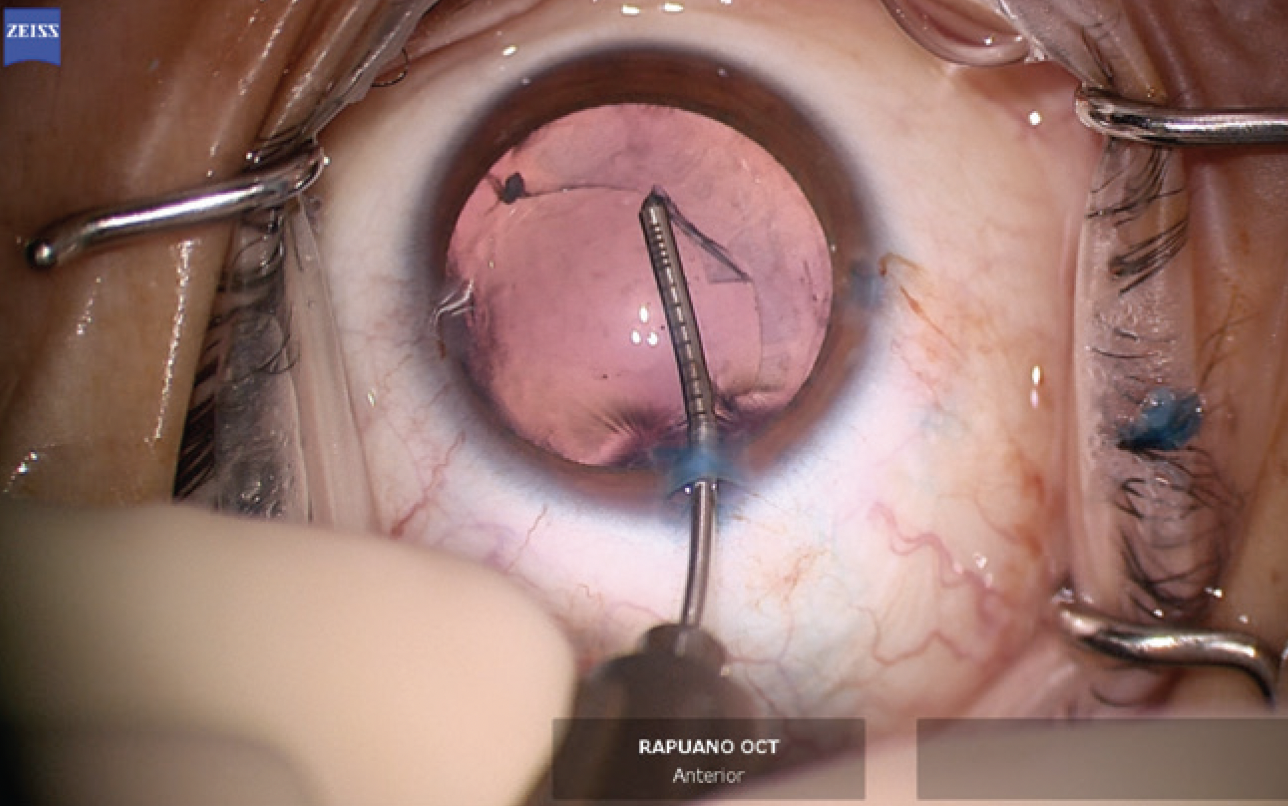
Figure 9. The primary capsulorhexis is enlarged with microcapsulorhexis forceps to prevent excessive capsular overlap of the IOL.
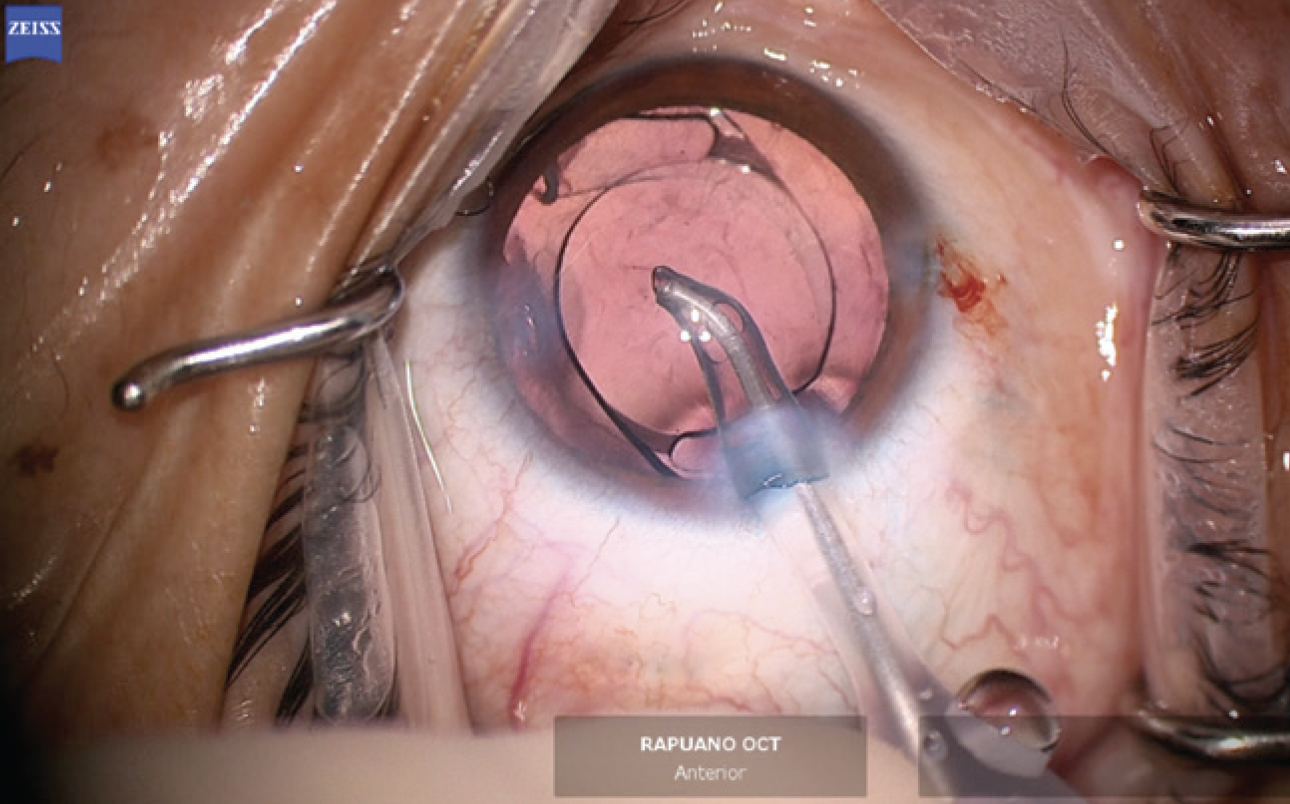
Figure 10. A one-piece acrylic IOL is placed in the capsular bag. Special care is taken to prevent exposure of the haptics by covering them with anterior capsular leaflets.
One day after surgery, the patient’s uncorrected distance visual acuity was 20/80 OD. At 1 month, his uncorrected distance visual acuity was 20/40 OD, which did not improve with pinhole testing or refraction. The patient managed well with a contact lens on the left eye. He and his family were excited about the vision he had recovered. A follow-up visit was scheduled for his next break from school.