Ocular surface disease is well known to be a major cause of visual disability. While an array of options are available for treatment, there always seems to be a place for more, especially when they fulfill persisting unmet needs.
In an episode where they explored the expanding options for treating dry eye disease (DED), John Hovanesian, MD, and Dr. Singh stressed the importance of identifying and treating ocular surface disease in any patient, but particularly for those exploring surgical options, be they cataract or refractive.
“It’s a must-treat condition—if we don’t address it, it will cause problems with the outcome,” Dr. Hovanesian said.
Amniotic Membrane for Severe DED
Amniotic membranes are typically thought of in the context of treating chronically symptomatic ocular surface disease, such as herpes simplex and neurotrophic keratopathy, where the surface doesn’t heal despite advanced treatments. However, according to a study by McDonald et al, they may have utility in refractory DED cases in which other treatments have failed.1 In the study, outcomes from 94 eyes of 84 patients from 10 clinical sites with severe DED treated with a cryopreserved amniotic membrane (CAM) were retrospectively reviewed. The affected eyes received a CAM for 5.4±2.8 days, and they were followed for 3 months. Clinical manifestations and comorbidities were common among eyes in the study, including superficial punctate keratitis (86%), filamentary keratitis (13%), exposure keratitis (19%), neurotrophic keratitis (2%), and corneal epithelial defect (7%). The study’s primary outcome was the change in patients’ dry eye workshop (DEWS) scores following the treatment (Figure 2).
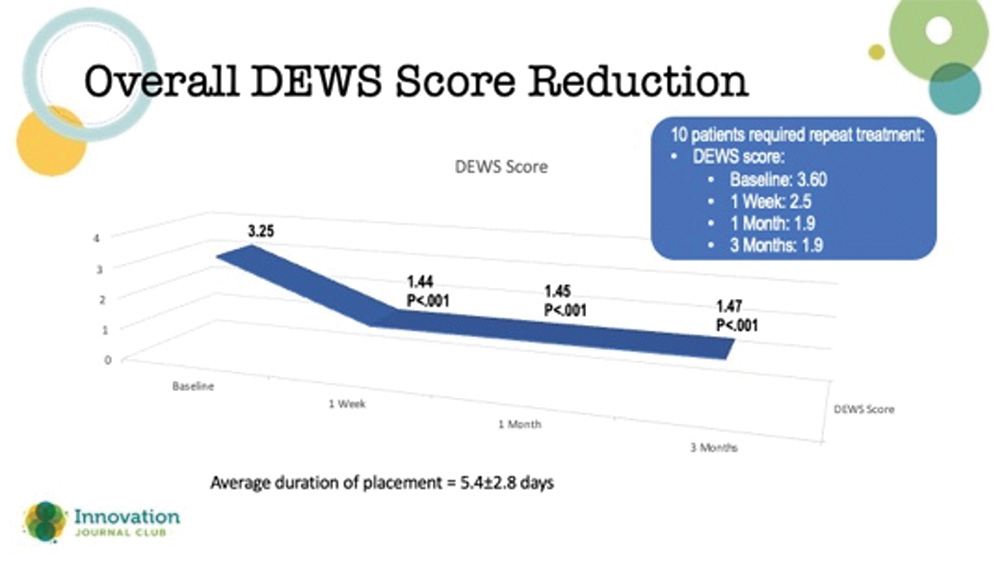
Figure 2. Reduction in DEWS score out to 3 months after use of an amniotic membrane for the treatment of refractory dry eye disease.
Dr. Hovanesian said that it was impressive that the effects of the CAM treatment did not wane in 3 months. He added that amniotic membranes can be used more than once if a patient’s corneas start to regress over time. Typically, these membranes are left on the eye for a few weeks to promote healing.
“The anti-inflammatory property is not just healing the cells that are defective, it's keeping them healed. It keeps the ocular surface in a homeostatic state for quite a long time,” Dr. Hovanesian said.
Dr. Singh commented that, when it comes to treating DED, symptomatology drives his decision-making. Dr. Hovanesian agreed, adding that one of the most telling symptoms for him is intermittent blurring: “Often, this symptom indicates a problem with the ocular surface,” he said.
MIEBO: An Unpreserved, No-Vehicle Topical Drop to Treat MGD-Associated DED
Dr. Hovanesian and Dr. Singh next discussed MIEBO (perfluorohexyloctane ophthalmic solution; formerly known as NOV03), the new preservative-free treatment from Bausch + Lomb that is indicated for the signs and symptoms of DED associated with meibomian gland dysfunction (MGD). MIEBO works by reducing tear evaporation from the ocular surface. “Evaporation happens because there's no hydrophobic layer to prevent evaporation of the aqueous, and the mucin layer becomes diminished when there's a disruption in the aqueous layer,” Dr. Hovanesian explained.
He described how MIEBO’s FDA approval stemmed from two 57-day, multicenter, randomized, double-masked, saline-controlled studies, GOBI and MOJAVE,2,3 wherein investigators randomized more than 1,200 patients with a history of DED and clinical signs of MGD to receive either perfluorohexyloctane or hypotonic saline. “This was well-done science,” said Dr. Hovanesian.
There were two primary endpoints in these studies: (1) a change from baseline in total corneal fluorescein staining (tCFS) at 8 weeks, and (2) patients’ ocular dryness scoring on the Visual Analog Scale (VAS). Results from both studies showed that relief of patients’ symptoms began as early as day 15 and continued through day 57, plus a significant reduction in dryness scores on the VAS (Figure 3).
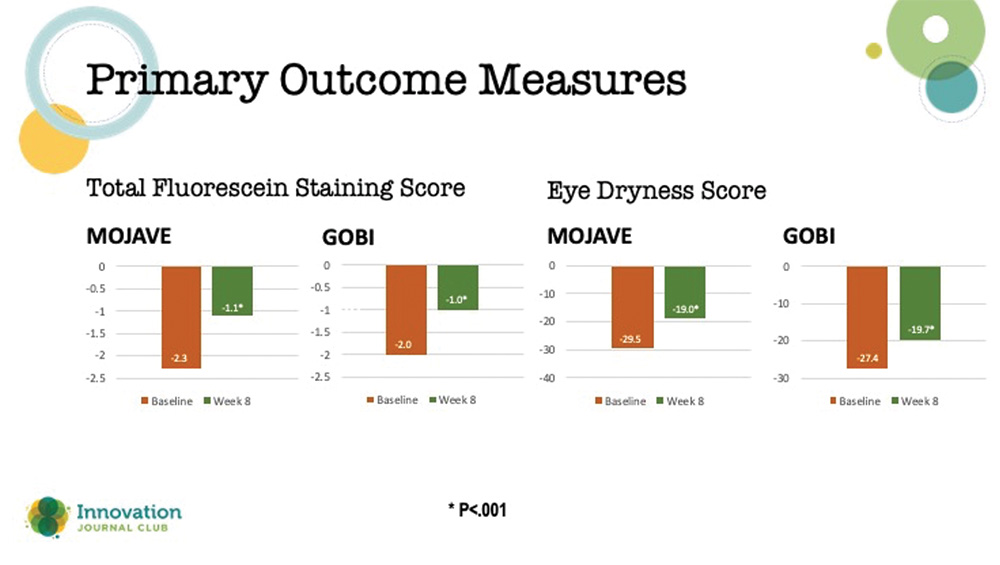
Figure 3. Primary outcome measures in the two phase 3 studies on MIEBO.
Dr. Hovanesian stressed that perfluorohexyloctane is a highly dispersive compound with both hydrophillic and lipiphillic components that form a monolayer over the eye. The molecule requires a very small drop size: 15–20 microliters compared to the 50-microliter size of a typical topical eye drop. “I'm excited, because this is an entirely different kind of approach to dry eye.”
He was also impressed with MIEBO’s safety profile. “We saw a very low rate of adverse events of blepharitis, about 1.6%. And in the phase 3 GOBI study, we saw a 9.6% rate of AEs compared to 7.5% with the saline group.”2,3 In his own practice, Dr. Hovanesian said that his patients’ tolerability of MIEBO has been excellent.
In conclusion, Dr. Hovanesian said that, although some DED patients will continue to need immunomodulators and anti-inflammatories because of their different mechanisms of action, there will be some individuals for whom MIEBO could replace these treatments, and be better tolerated.
1. McDonald MB, Sheha H, Tighe S, et al. Treatment outcomes in the DRy Eye Amniotic Membrane (DREAM) study. Clin Ophthalmol. 2018;12:677-681.
2. Tauber J, Berdy GJ, Wirta DL, et al. NOV03 for dry eye disease associated with meibomian gland dysfunction: results of the randomized phase 3 GOBI study. Ophthalmology. 2023;130(5):516-524.
3. Sheppard J, Kurata F, Epitropoulos AT, et al. NOV03 for signs and symptoms of dry eye disease associated with meibomian gland dysfunction: the randomized phase 3 Mojave study. Am J Ophthalmol. 2023;252:265-274.

