
Assessing BCVA alone does not capture the effect of geographic atrophy (GA) on visual function. With GA, patients often have sparing of central visual acuity, but they still experience profound deficits in visual acuity. With GA, poor low-light vision is a major patient complaint, and low luminance dysfunction predicts subsequent visual acuity loss in GA. The speed with which patients can read is another major complaint of GA patients, and it has substantial impact. The speed with which patients are able to read has an inverse correlation with GA lesion size. Microperimetry is another barometer of GA extent; it correlates GA progression with scotomatous points in the macular area.
Imaging Methods
There are several imaging methods available to quantify GA age-related macular degeneration (AMD), such as color fundus photography, autofluorescence, Spectral Domain (SD)-optical coherence tomography (OCT) and Swept Source (SS)-OCT. GA-AMD is a progressive disease; the assessment of GA from OCT images is an important clinical and research task as it provides valuable information regarding the evolution of the disease. OCT is one procedure that can verify progression of GA-AMD. Using SS-OCT, the device provides us a high-resolution study of the atrophic area, as this example shows (Figure 1). Hotlz et al. have published that fundus autofluorescence (FAF) imaging can identify GA subtypes that may predict the rate of lesion enlargement, as seen in this example (Figure 2).
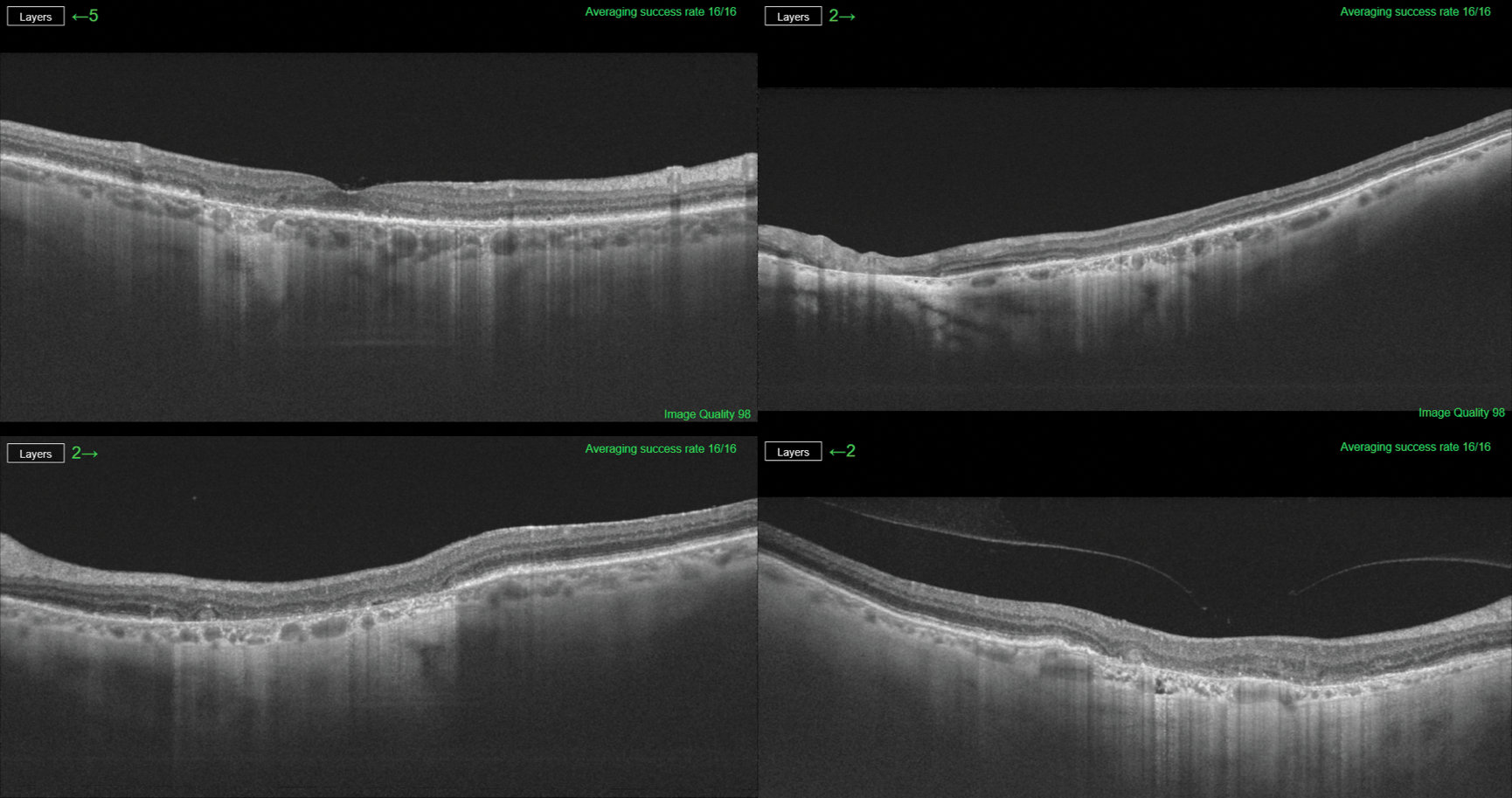
Figure 1. SS-OCT provides high resolution study of the atrophic area in a case of GA.
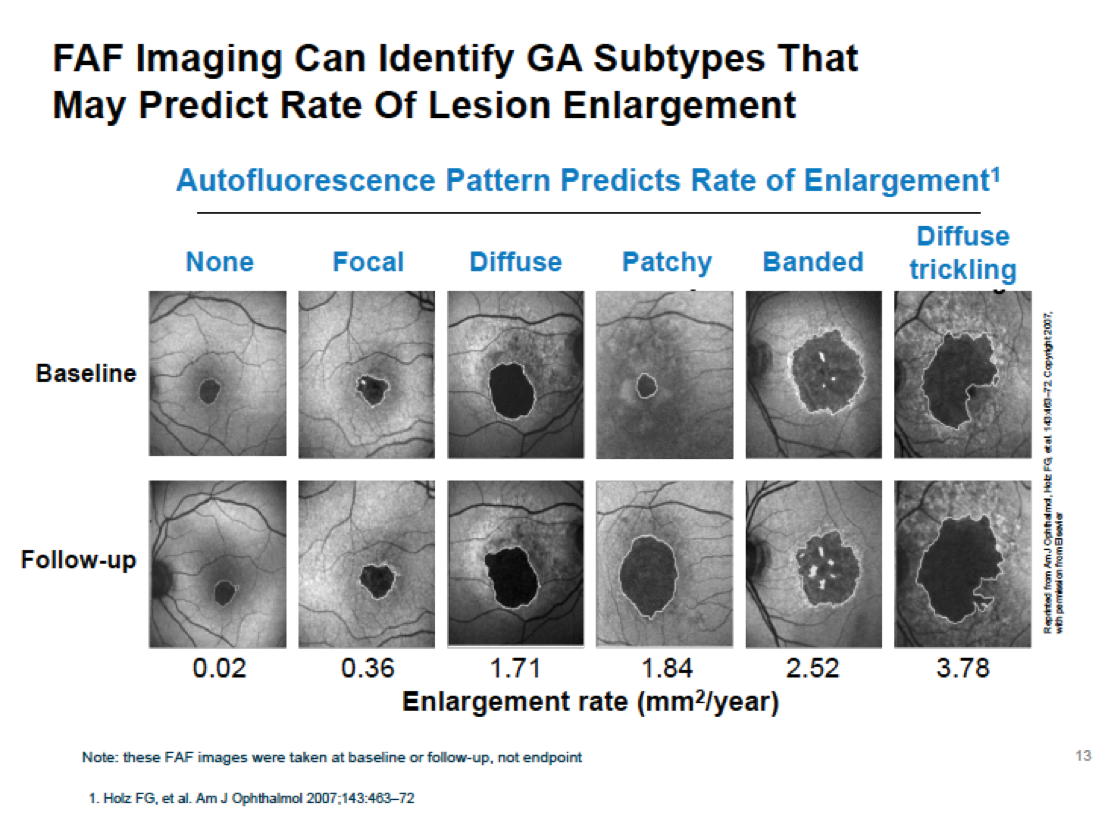
Figure 2. FAF imaging identifying GA subtypes that may predict the rate of lesion enlargement.
Automated GA-AMD Quantification
Due to the time consuming and subjective nature of manual image analysis, there is a need for reliable objective automated methods of image segmentation to obtain GA measures of both lesion perimeter and lesion area. The vast majority of previous studies examining GA, with imaging methods, have utilized manual measurement procedures, which have several imitations. They require subjective judgments; they can be too time consuming to be practical in a clinical setting; they can potentially be prone to bias; and they presented great difficulty because of the numerous data sets needed for statistical analysis.
For all of these reasons, new software was developed by Topcon (Japan) to reduce the complexity of compiling imaging data. In order to determine the accuracy and repeatability of the new software, we carried out a cross sectional, non-interventional study. The aim of the study was to evaluate this software’s ability to automate and simplify quantification of GA-AMD using SS-OCT.
Patients and Methods
- This cross-sectional, non-interventional study included 60 eyes from AMD patients, with GA and without previous choroidal neovascularization (CNV). They were scanned using a Triton SS-OCT (Topcon, Japan).
- The study of every eye included color fundus photography, autofluorescence imaging, OCT study with a 7x7 cube area with eye tracking, repeated three consecutive times, and five-line cross of 12 mm.
- Three observers independently determined the area and the perimeter at the FAF image as ‘gold standard’ measurement of the size of the GA area.
- They independently performed the corrections at the automatic segmentation of the atrophic area.
- All tasks were performed in a masked fashion.
- Inclusion criteria were GA-area associated to AMD without previous CNV, enough capacity to fixate the OCT test, the entire atrophic area must be included in 7x7 cube, and no opacities of the media.
- At the time of this presentation we had 42 eyes from 32 AMD patients (10 bilateral cases).
- There was 61% female (26/16); all were Caucasian.
- Median BCVA was 20/160, with a range of 20/40 to 20/400.
Study Aims:
- To study the reproducibility of the software by comparing the automatic area and the automatic perimeter between the three consecutive explorations.
- To determine the accuracy of the software by comparing the data obtained with manual measurements versus automatic measurements.
- To study the real efficacy of the software by comparing the corrected automatic measurements versus manual measurements.
Study Outcomes and Conclusion
- The new software allows us to automatically do the quantification of the GA area and perimeter associated to AMD.
- The image analysis is fast, objective, with a smaller coefficient of variation than manual procedures.
- The accuracy of the new software is better in regular atrophic lesions. It's necessary to improve its segmentation in irregular cases.
This study has been supported in part by a grant from the Spanish Ministry of Health, Instituto de Salud Carlos III, Red Temática de Investigación Cooperativa en Salud: "Prevención, detección precoz y tratamiento de la patología ocular prevalente, degenerativa, crónica” (RD16/0008/0021). Dr. José M. Ruiz-Moreno receives research support from Topcon.
1. Wong WL et al. The Lancet Glob Health. 2014;2:e106-e116.
2. AREDS Research Group. Arch Ophthalmol. 2001;119:1417-1436.



