Reproducible, precise methods generally produce predictable, excellent outcomes when combining laser surgery with phacoemulsification for cataract removal procedures. This article shares some of my surgical techniques with the Catalys (Abbott Medical Optics), many of which can be used with other platforms.
POSITIONING
Position the patient’s head to get the best possible exposure without interference from the Catalys’ liquid optic interface (LOI), the orbital rim, or the the nose. For example, if I am working on the right eye, then I tilt the patient’s head to the left. I place the LOI on the eye by putting the rim of the cup on the lower lid, pushing down, and then pulling the upper lid upward. For surgeries requiring arcuate incisions, I use the standard-sized LOI, which allows me to clearly see the incision area, limbus, and any guide marks on the sclera. If I will not be performing any corneal incisions, I prefer the smaller interface because I find it is easier to maneuver between the eyelids and center on the cornea as compared to the larger LOI.
CUSTOMIZED SETTINGS
Consistently achieving a clean capsulotomy largely depends on selecting optimal laser settings and minimizing the patient’s movement. When the cornea is clear and there is no movement, the laser will cut a perfect capsulotomy every time. One of the settings the surgeon may want to change from the factory standard is vertical spacing. When too close together, it can cause the pulses to hit the same area of the capsule twice, resulting in capsular slivers or double tracks and potentially creating a radial point of weakness. A major advantage of increasing the vertical spacing is the decrease in the treatment time of the capsulotomy to less than 1 second.
I use a vertical spacing of 15 µm or greater and an incision depth of 500 µm. Timing the capsulotomy relative to the point in the patient’s breathing cycle when there is the least movement is another pearl. Even with the vacuum on, there is still microscopic movement of the eye detectable only on the monitor. If the movement is rhythmic, it is probably due to breathing. Rather than risk a large movement when patients inhale deeply to hold their breath or aggravate problems in individuals with pulmonary disease, I time the capsulotomy treatment to the end of exhalation. Timing, together with the increase of vertical spacing and shorter treatment time, helps reduce the likelihood of overlapping laser pulses.
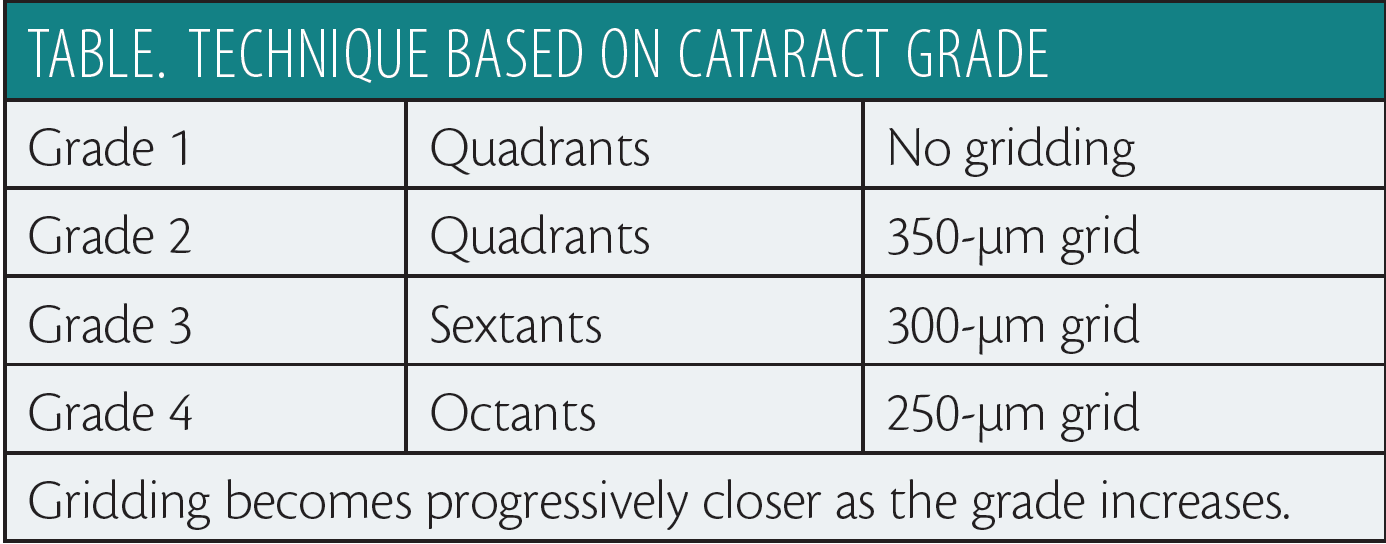
I base emulsification settings on the grade of cataract, which determines how I want to chop the lens, the grid pattern I want to use, and how much energy I want to put into softening the lens. Regardless of the method, dividing the lens in two is the most important aspect of reducing phaco energy. I have developed two techniques to accomplish this step. First, I attempt endolenticular viscodissection. After placing Healon (Abbott Medical Optics) in the anterior chamber, I direct a 30-gauge cannula on a Healon syringe into the center of the lens and inject the OVD (Figure 1). In most cases, this technique will split the lens, confirmed when I see the OVD pass posterior to the lens. If the crystalline lens is too dense for this approach, I use a bimanual technique with a Bechert fork and a Scott Femto Chop (Crestpoint Ophthalmics; Figure 2). Along the segment line, the chop is placed distally at the capsulotomy’s edge, and the fork is placed proximally. The chop is drawn toward the center of the lens, and the fork is pushed to the center. When the instruments meet in the center, I push them away from each other in the perpendicular horizontal plane, thus separating the lens into two halves. I have found that almost all dense cataracts can be divided in this way. In my hands, prechopping the lens permits the safe use of the highest aspiration level, and the laser chop instrument helps me to manipulate pieces to the central safety zone and protects the posterior capsule during removal of the last portions of the lens.
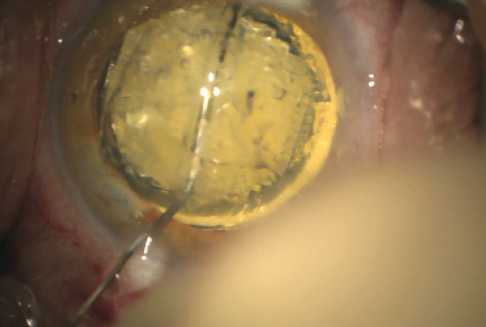
Figure 1. Using a 30-gauge cannula, an ophthalmic viscosurgical device (OVD) is injected into the dividing line created by the femtosecond laser, resulting in endolenticular viscodissection that assists in dividing the cataract lens into two halves.
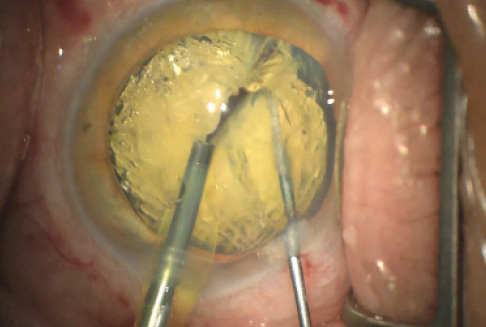
Figure 2. The curve of the Scott Femto Chop is useful for further cracking and disassembly of the laser-treated cataract.
IRRIGATION/ASPIRATION
Using a Venturi sweep technique, I initiate I/A subincisionally (Table). The constant aspiration pulls the cortex to the tip and allows me to sweep the aspiration port along the capsular edge without going very far under the capsulotomy. When removing the lens, I keep the phaco tip in the center and use high aspiration in the Venturi mode to allow the pieces to come to the tip. I prefer removing the lens in segments to prevent lens fragments from flying around the anterior chamber and potentially causing endothelial damage and to keep pieces from being retained between the iris and the capsule. After inserting the IOL, when aspirating the viscoelastic, I direct the aspiration port in Venturi mode along the edge of the capsulotomy (slightly anterior to the capsulotomy) to ensure that any fragments posterior to the iris come to the tip.
ARCUATE INCISION PLACEMENT
Accurate placement of the arcuate incision begins with centration of the capsulotomy based on the center of the scanned capsule and not in relation to the pupil. I find that this strategy also results in the best IOL centering and that it is a reproducible way to position intrastromal arcuate incisions. Patients sit up and stare straight ahead when I place marks on the eye. I use a pendulum-weighted marking device (Scott Limbus Marker; Crestpoint Ophthalmics) that always stays level with three points of limbal contact at 3, 9, and 6 o’clock.
As surgeons learn from our experience, they will continue to improve the art of laser cataract surgery.
Wendell J. Scott, MD
• in practice at Mercy Eye Specialists in Springfield, Missouri
• (417) 820-9393; wendell.scott@mercy.net
• financial disclosure: consultant to Abbott Medical Optics