CASE PRESENTATION
A 70-year-old white man presented for cataract surgery. His past ocular history was significant for four-cut radial keratotomy (RK) in his right eye and four-cut RK with T-cut in his left eye in 1996. Additionally, the patient was post–selective laser trabeculoplasty for primary open-angle glaucoma and was using a prostaglandin analogue drop at bedtime in each eye. The results of visual field testing and tonometry had remained stable for 9 years. Temporal and inferior thinning of the optic nerve was evident, with a cup-to-disc ratio of 0.75 OU. Further, the patient had a history of central retinal vein occlusion that was currently stable and a mild epiretinal membrane in his right eye.
Examination revealed a posterior subcapsular and nuclear sclerotic cataract in each eye. The patient’s BCVA was 20/60 OD with a refraction of -2.00 +1.75 x 14º and 20/50 OS with a refraction of -1.50 +1.00 x 30º. After an extensive workup, the patient underwent uneventful laser cataract surgery and implantation of a Tecnis Toric IOL (Johnson & Johnson Vision), with guidance from intraoperative aberrometry (ORA System, Alcon), in his right eye. Two weeks later, he underwent uneventful laser cataract surgery and implantation of an enVista IOL (Bausch + Lomb), also guided by intraoperative aberrometry, in his left eye.
One month after surgery, the patient had an uncorrected distance visual acuity of 20/25 and a corrected distance visual acuity of -0.25 +0.50 x 41º = 20/20 in the patient’s right eye. He had an uncorrected distance visual acuity of 20/30 that corrected to 20/25+2 with -0.25 D sphere in his left eye.
During a trip out of state, the patient’s postoperative course was complicated by a kick to his right eye by a grandchild. The injury resulted in a dehisced wound and iris prolapse. The eye was repaired and the iris reposited without any postoperative infection. On followup with me and in further discussion, he stated that the difference in visual acuity between his eyes necessitated his use of reading glasses at night. On further questioning, he clarified that the discrepancy had been present since his original RK procedures.
Because of visual fluctuation and refractive instability, the patient underwent ray-tracing aberrometry with the iTrace (Tracy Technologies) at different times in the morning and afternoon. In each eye, testing revealed variations from -0.25 to 2.50 D in sphere, from 1.00 to 1.75 D in cylinder, from 49º to 74º in axis, from 20/25 to 20/70 in uncorrected near visual acuity, and from 20/20 to 20/25 in corrected distance visual acuity (Figures 1 and 2).
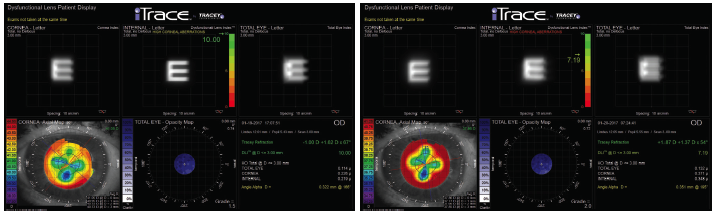
Figure 1. The results of ray-tracing aberrometry in the patient’s right eye performed at 5 pm (left) and 7 am (right).
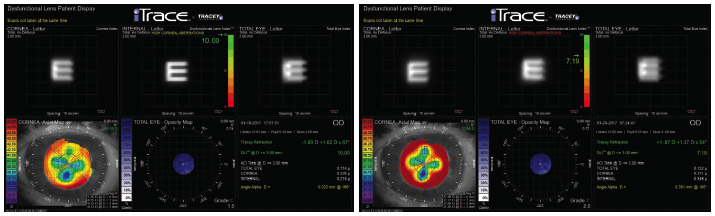
Figure 2. The results of ray-tracing aberrometry in the patient’s left eye performed at 5 pm (left) and 7 am (right).
How would you proceed?
—Case prepared by
P. Dee G. Stephenson, MD, FACS

KENNETH A. BECKMAN, MD
I am unsure how much time has elapsed since cataract surgery. Nevertheless, based on postoperative visual acuity and refraction, it appears that if the correct IOL was implanted. The problem is fluctuating vision from an unstable cornea. In my experience, reducing the IOP tends to temper visual fluctuation, at least somewhat, in most post-RK patients. This patient has a history of glaucoma and is already taking IOP-lowering medication. In general, I like to start post-RK patients on brimonidine. In addition to the decrease in IOP, these patients benefit from the drug’s miotic effect.
If not much time has passed since the cataract and repair surgeries, then I would hold off on further surgical intervention because time may be the greatest ally in this situation. The patient may require different glasses in the morning and evening. Scleral contact lenses might also help; they can compensate for subtle variations in corneal curvature.
Years ago, I achieved modest success with a lasso suture in patients who were overcorrected by RK, but in this case the issue is instability rather than overcorrection. If the conservative measures mentioned earlier failed to resolve the problem, I would consider CXL. I do not believe the risk of gaping incisions with CXL would be high, and the procedure might stabilize the cornea. Although the eye might not have an emmetropic result, glasses or contacts might be sufficient for refractive correction.

KAROLINNE MAIA ROCHA, MD, PhD
In the 1990s, many myopic patients underwent RK to minimize their dependence on glasses, and now they are returning to ophthalmic practices for lens surgery. Diurnal variation in vision is common in this population. This case demonstrates the hyperopic shift in the morning and refractive fluctuation throughout the day that post-RK patients typically experience after cataract surgery. These phenomena can occur during the first few months after cataract surgery, and they are related to changes in the tear film and in IOP during the day.
The shift in higher-order aberrations during the day in this case is interesting. Usually, glasses and soft contact lenses are inadequate for managing fluctuations in lower- and higher-order aberrations. Rigid gas permeable contact lenses are often the best option for these patients because this corrective modality can compensate for irregular astigmatism.
Although transepithelial CXL seems to be a safe and effective method of reducing diurnal fluctuations, the central corneal flattening that the procedure produces can worsen a patient’s hyperopic shift.1-3 Because the RK incisions could continue to change, and because the progressive hyperopic shift may continue over time, CXL could be a great option for stabilizing the cornea in this patient.

PRIYANKA SOOD, MD
In cases such as this one, it is important to help patients understand their complex condition in a way that shows empathy for their situation while also setting realistic expectations regarding outcomes. A large percentage of patients have long-term refractive instability after RK. Prior to cataract surgery, I inform all of my post-RK patients that their refractive outcome may continue to change for a few months after the procedure. Edema at the time of cataract surgery can lead to flattening of the central cornea, which can leave patients with a hyperopic result immediately after surgery. As the edema resolves over time, they experience a myopic shift, and their refraction stabilizes.
In this case, the patient achieved an excellent outcome with a toric IOL but then sustained a traumatic injury. He has undergone multiple operations in a short amount of time, so I would allow his cornea to stabilize before considering another possible surgical intervention. In the interim, given the daily fluctuations, I would have him fitted for a scleral contact lens. Scleral lenses are an excellent option because they vault over the cornea and are therefore not affected by changes in corneal curvature. They are also comfortable to wear.4
Because many patients underwent RK to be free of contact lenses, this patient may not wish to wear a scleral contact lens long term. Some studies have shown that CXL can help to stabilize a fluctuating post-RK cornea.2,5 At present, it is not possible to predict how much stability or possible flattening CXL will produce, which could mean a shift in the refractive error, but the patient might be happier with refractive stability even if his visual acuity changed. If CXL did leave the patient with a refractive error, PRK could be considered once refractive stability was achieved.6
Whatever plan of action is selected, it will be imperative to explain to the patient that it will be a journey he and his surgeon will share and that the final destination is uncertain.

WHAT I DID: P. DEE G. STEPHENSON, MD, FACS
In April 2017, I referred the patient to a colleague in South Florida for CXL. Since the procedure, the cornea has exhibited stability, and the iTrace has found less variation over the course of the day. Specifically, the variation in spherical equivalent decreased from a range of -0.19 to 2.50 D before CXL to a range of 0.37 to 0.75 D at 1 year after CXL in the patient’s right eye (Figures 3 and 4). The patient rarely uses glasses, but, when he does, he relies on a correction of 1.00 D for driving late at night. He reports loving his vision for playing golf.
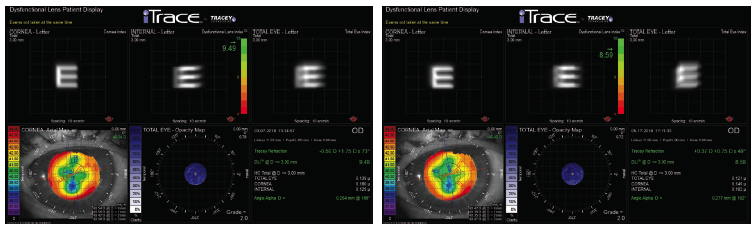
Figure 3. After CXL, the results of ray-tracing aberrometry in the patient’s right eye performed at 1:30 pm (left) and 5 pm (right).
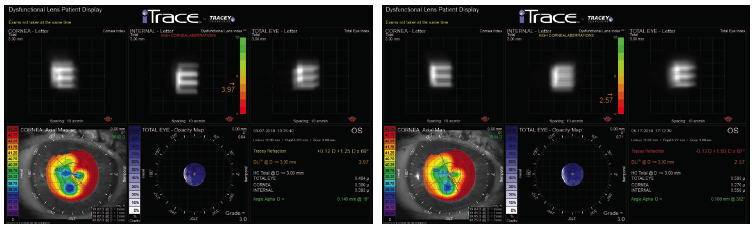
Figure 4. After CXL, the results of ray-tracing aberrometry in the patient’s left eye performed at 1:20 pm (left) and 5 pm (right).
The case has prompted me to ask more questions of my post-RK patients, and it has made me wonder whether CXL should be advised for every post-RK patient as well as for anyone with an unstable cornea after laser vision correction.
1. Elbaz U, Yeung SN, Ziai S, et al. Collagen crosslinking after radial keratotomy. Cornea. 2014;33(2):131-136.
2. Fuentes-Páez G, Castanera F, Gómez de Salazar-Martinez R, et al. Corneal cross-linking in patients with radial keratotomy: short-term follow-up. Cornea. 2012;31(3):232-235.
3. Roberts CJ, Dupps WJ Jr. Biomechanics of corneal ectasia and biomechanical treatments. J Cataract Refract Surg. 2014;40(6):991-998.
4. Parminder A, Jacobs DS. Advances in scleral lenses for refractive surgery complications. Curr Opin Ophthalmol. 2015;26(4):243-248.
5. Elbaz U, Yeung SN, Ziai S, et al. Collagen crosslinking after radial keratotomy. Cornea. 2014;33(2):131-136.
6. Lin DT, Holland S, Tan JC, Moloney G. Clinical results of topography-based customized ablations in highly aberrated eyes and keratoconus/ectasia with cross-linking. J Refract Surg. 2012;28(11 suppl):S841-S848.




