


Technological advances and clinical experience have shifted the paradigm of keratoconus management from disease stabilization to comprehensive, customized visual rehabilitation—even for advanced keratoconus. The approach consists of creating a treatment plan tailored to each cone, mainly through a three-pronged strategy. First, when necessary, the cone is debulked with intrastromal corneal ring segments (ICRSs), preferably allogenic models.1,2 Second, the results are fine-tuned with customized surface ablation targeting higher-order aberrations (HOAs). Third, residual lower-order aberrations (LOAs) are addressed with tissue-sparing techniques such as spectacles or soft contact lenses and the implantation of a phakic or small-aperture IOL. The last step helps minimize tissue ablation, maintain stromal integrity, and avoid refractive overcorrection.
DEBULKING THE CONE
Ring segments. ICRSs regularize the corneal surface and achieve good visual and topographic results.3,4 Corneal allogenic intrastromal ring segments (CAIRS), an alternative to PMMA segments, have demonstrated comparable effectiveness (Figure 1). Allogenicity enhances the segments’ tolerability in the stroma, minimizing the risk of corneal melt and anterior stromal necrosis.1 CAIRS may therefore be inserted at a shallower depth in the stroma to induce a more pronounced effect (click here for video demonstrations).
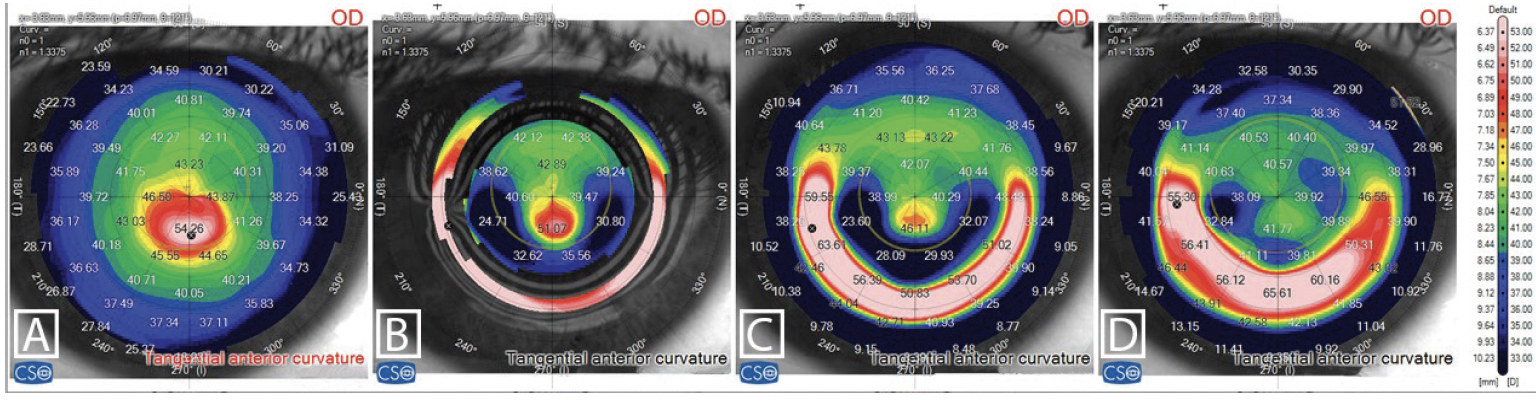
Figure 1. Tangential anterior curvature maps: preoperatively (A), 1 week (B) and 1 month (C) following CAIRS implantation, and following customized ablation without MMC application (D).
Allogenic segments are either prepared by a surgeon or manually precut by an eye bank. An example of the latter is KeraNatural (VisionGift). When prepared by a surgeon, CAIRS can be cut with a manual double-bladed Jacob trephine (Madhu Instruments) or by an automated software on a femtosecond laser platform by means of an artificial chamber (Figure 2). The latter technique allows accurate and repeatable creation of asymmetric segments to target certain keratoconic phenotypes; the width and thickness of each end and at the center of a segment can be specified separately. Eventually, eye banks may adopt laser-cut CAIRS and provide customized segments, making the maintenance of an extensive inventory of tissue unnecessary.

Figure 2. A slit-lamp examination showing laser-cut CAIRS in place.
By debulking the cone, ICRSs make the host cornea amenable to further treatment that would otherwise be impossible owing to the eye’s topographic characteristics.
Lenticule implantation. Advanced keratoconus can be managed with stromal lenticule implantation. The technique can safely improve the vision, topography, and refraction of keratoconic eyes mainly through a compensatory increase in corneal thickness that reduces keratometric values and debulks the cone.5-9
Crosslinking. Conventional CXL is widely used to slow the progression of keratoconus but with a modest flattening effect.10 Theoretical studies by Sinha Roy and Dupps11 prompted the development of CXL techniques that preferentially stiffen areas of high focal weakness and redistribute biomechanical stress. For example, customized CXL with the Mosaic System (Avedro) can stabilize and flatten the cornea.12,13
In collaboration with Farhad Hafezi, MD, PhD, FARVO, and The Elza Institute, we are developing a second-generation customized CXL approach called phototherapeutic keratectomy (PTK)–assisted customized epithelium-on CXL (PACE).14 The PACE technique consists of three steps:
- An excimer laser is used to shave the epithelium over the cone;
- Riboflavin penetration enhancers are administered; and
- Epithelium-on CXL without supplemental oxygen is performed.
A gradient of riboflavin concentration subsequently forms, and the highest levels are observed over the cone. Eventually, selective flattening occurs over this area (F.H., unpublished data, June 2023). PACE may become another tool for debulking the cone.
CXL can make it possible to perform concomitant or sequential customized ablations and thus fine-tune the major (but rough) regularization achieved with ICRSs.
FINE-TUNING RESULTS
Haze and timing. CXL is an effective method for halting keratoconus progression, but the procedure alone may not provide adequate visual rehabilitation to patients who require but are unable to tolerate rigid gas permeable lenses. Customized surface ablation, introduced with the Athens protocol,15 improves patients’ visual results. The main considerations with customized PRK in these eyes are the potential development of haze and procedural timing with respect to CXL (ie, combined vs sequential).
The literature contains conflicting evidence on the amount of haze that develops after combined CXL and customized ablation versus a sequential approach. The variation observed across studies may be attributable to the extent of tissue ablation, which appears to be in direct proportion to the induction of haze. Stromal ablation should therefore be minimal, targeting mainly HOAs.
The use of mitomycin C (MMC) can also contribute to the development of haze. We evaluated eyes with an OCT-based machine learning algorithm and found haze to be more significant following CXL performed with versus without MMC.16 It is possible that the combined actions of MMC and CXL on the keratocyte population trigger a substantial release of cytokines and chemokines, which could lead to the formation of haze.
As for combined versus sequential CXL and PRK, current scientific evidence does not favor one approach over the other. Clinical experience suggests that the choice of approach depends on patient selection and the surgeon’s objectives. For instance, an eye with a mild cone, relatively good corrected distance visual acuity (CDVA), and minimal symptoms could benefit from CXL alone and potentially later sequential treatment, whereas an eye with a moderate cone, suboptimal CDVA, and visual symptoms is more likely to benefit from combined treatment.
Targeting aberrations. The customized ablation procedure should target HOAs and only those LOAs embedded in them. Recently developed algorithms on excimer laser platforms such as the Amaris (Schwind eye-tech-solutions) calculate the corresponding refraction to maximize the correction of HOAs with the least possible amount of tissue ablated in depth and volume. This minimizes overcorrection and saves precious stromal tissue. The suggested refraction can be tweaked according to the refraction, topography, and clinical situation. With this strategy, the optical zone is decoupled from the ablation depth. The optical zone may therefore be enlarged beyond the 5.5 mm suggested by the Athens protocol.
Our general rule for customized ablation on keratoconic eyes is the following: The less, the better. Residual LOAs are addressed with other tissue-sparing optical and surgical modalities (discussed later).
Selective treatment of aberrations and total corneal wavefront-guided treatment. The advent of pyramidal aberrometers with large dynamic ranges and high resolution allows the measurement of complex ocular wavefront patterns, including those found in eyes with corneal ectasia.
Corneal wavefront-guided (CWG) treatment builds on topography-guided treatment. Topography is distilled into a wavefront map, and the surgeon chooses which aberrations to treat, thereby reducing the volume and depth of tissue ablation. Like topography-guided procedures, CWG treatment is limited to the anterior surface and cannot account for posterior curvature, which is at a negative refractive meniscus and, in keratoconic eyes, often helps negate anterior curvature aberrations. Ocular wavefront-guided (OWG) treatment, especially when performed with high–dynamic range aberrometers, can address the total corneal wavefront (anterior and posterior) while minimizing both the amount of tissue ablated and overcorrection. To qualify for OWG treatment, the mesopic or scotopic pupil must be substantial enough to ensure a large optical zone, the eye should not have a cataract, and no major intraocular aberrations should be present.
In one study, 30% to 40% less tissue was ablated with OWG compared to CWG treatment for keratoconus with the Amaris.17 The recent availability of total CWG treatment on this and other excimer laser platforms that use high-definition anterior segment OCT allows both anterior and posterior corneal wavefront errors to be corrected (Figure 3).

Figure 3. Topography of a 25-year-old man with a history of CXL. Dual Placido and anterior segment OCT (MS39, CSO) show anterior, posterior, and total corneal wavefronts (A). Anterior corneal coma is 0.86 @ 100º, posterior coma is 0.41 @ 285º, and total coma is 0.43 @ 97º (B). Ocular wavefront aberrometry with a pyramidal aberrometer (Peramis, CSO) shows total ocular coma of 0.46 @ 92º (C). Topography-guided or CWG laser ablation would treat the anterior coma and expose the posterior coma, resulting in 0.41 @ 285º of total coma, the same amount as preoperatively but on the opposite axis. OWG treatment of the total corneal wavefront would be more effective because it would correct the total corneal coma. Topography shows improvement after OWG treatment (D). Some anterior corneal coma (0.39 @ 103º) remains to offset the posterior coma (0.44 @ 283º), resulting in zero total corneal coma (E).
Our experience. We generally favor customized PRK for the treatment of up to 0.80 D of coma in an eye with a 6-mm pupil. In an eye with more significant aberrations, ICRSs or allogenic segments would be inserted first, and customized CXL or PACE would be performed, if indicated, before the result is fine-tuned with customized surface ablation.
Highly accurate epithelial thickness maps also make PTK a feasible option for keratoconic eyes. These maps can guide transepithelial PTK for selective stromal tissue ablation over the cone, where the epithelium is the thinnest. This strategy can partially treat coma while minimizing tissue removal. Such a tailored approach is most suitable for patients who have limited stromal tissue (ie, too little for PRK) and wish to improve their already relatively good CDVA.
ADDRESSING RESIDUAL LOAS
After the cone has been debulked and HOAs treated, residual LOAs may be managed with tissue-sparing options such as spectacles, soft contact lenses, and phakic or small-aperture IOL implantation. Pinhole IOLs can extend depth of focus by combining small-aperture technology with a monofocal IOL such as the IC-8 Apthera (Bausch + Lomb). The Xtra Focus (Morcher) is a pinhole sulcus lens implant that is meant to be inserted in a piggyback configuration over another IOL, including a toric model.
CONCLUSION
A dramatic shift has occurred in keratoconus management. The emphasis has moved from traditional corneal surgery to therapeutic refractive surgery owing in part to diagnostic advances such as the development of pyramidal aberrometry and OCT-derived tomography and to highly individualized treatments, including conventional and customized CXL, customized surface ablation, synthetic and allogenic intrastromal segments, and phakic and small-aperture IOLs.
Refractive surgeons are better positioned than ever to offer safe, innovative, and efficacious care to patients with keratoconus. Treatment must be tailored to each eye to halt disease progression and provide visual rehabilitation while minimizing tissue removal.
1. Jacob S, Patel SR, Agarwal A, Ramalingam A, Saijimol AI, Raj JM. Corneal allogenic intrastromal ring segments (CAIRS) combined with corneal cross-linking for keratoconus. J Refract Surg. 2018;34(5):296-303.
2. Haciagaoglu S, Tanriverdi C, Keskin FFN, Tran KD, Kilic A. Allograft corneal ring segment for keratoconus management: Istanbul nomogram clinical results. Eur J Ophthalmol. Published online December 4, 2022. doi:10.1177/11206721221142995
3. Kymionis GD, Siganos CS, Tsiklis NS, et al. Long-term follow-up of Intacs in keratoconus. Am J Ophthalmol. 2007;143(2):236-244.
4. Alió JL, Shabayek MH, Artola A. Intracorneal ring segments for keratoconus correction: long-term follow-up. J Cataract Refract Surg. 2006;32(6):978-985.
5. Mastropasqua L, Nubile M, Salgari N, Mastropasqua R. Femtosecond laser–assisted stromal lenticule addition keratoplasty for the treatment of advanced keratoconus: a preliminary study. J Refract Surg. 2018;34(1):36-44.
6. Ganesh S, Brar S. Femtosecond intrastromal lenticular implantation combined with accelerated collagen cross-linking for the treatment of keratoconus—initial clinical result in 6 eyes. Cornea. 2015;34(10):1331-1339.
7. Pedrotti E, Bonacci E, Fasolo A, et al. Meniscus-shaped stromal lenticule addition keratoplasty for corneal regularization and thickening in advanced keratoconus. Cornea. 2023;42(10):1221-1228.
8. Jadidi K, Mosavi SA. Keratoconus treatment using femtosecond-assisted intrastromal corneal graft (FAISCG) surgery: a case series. Int Med Case Rep J. 2018;11:9-15.
9. Doroodgar F, Jabbarvand M, Niazi S, et al. Customized stromal lenticule implantation for keratoconus. J Refract Surg. 2020;36(12):786-794.
10. O’Brart DP. Corneal collagen cross-linking: a review. J Optom. 2014;7(3):113-124.
11. Sinha Roy A, Dupps WJ Jr. Patient-specific computational modeling of keratoconus progression and differential responses to collagen cross-linking. Invest Ophthalmol Vis Sci. 2011;52(12):9174-9187.
12. Seiler TG, Fischinger I, Koller T, Zapp D, Frueh BE, Seiler T. Customized corneal cross-linking: one-year results. Am J Ophthalmol. 2016;166:14-21.
13. Sachdev GS, Ramamurthy S, B S, Dandapani R. Comparative analysis of safety and efficacy of topography-guided customized cross-linking and standard cross-linking in the treatment of progressive keratoconus. Cornea. 2021;40(2):188-193.
14. Hafezi F, Torres-Netto EA, Hillen M. Expanding indications for corneal cross-linking. Curr Opin Ophthalmol. 2023;34(4):339-347.
15. Kanellopoulos AJ, Asimellis G. Keratoconus management: long-term stability of topography-guided normalization combined with high-fluence CXL stabilization (the Athens protocol). J Refract Surg. 2014;30(2):88-93.
16. Awwad ST, Chacra LM, Helwe C, et al. Mitomycin C application after corneal cross-linking for keratoconus increases stromal haze. J Refract Surg. 2021;37(2):83-90.
17. Gore DM, Leucci MT, Anand V, Fernandez-Vega Cueto L, Arba Mosquera S, Allan BD. Combined wavefront-guided transepithelial photorefractive keratectomy and corneal crosslinking for visual rehabilitation in moderate keratoconus. J Cataract Refract Surg. 2018;44(5):571-580.




