
In 2008, apprehensions about the future of LASIK and other laser vision correction (LVC) techniques emerged. Fast forward to 2023, and concerns about LVC are again being voiced, echoed by mainstream sources.
A recent cover of a European ophthalmic trade publication posed the following provocative question: “Is this the end of LASIK?”1 The title of a symposium at the 2023 Ophthalmology Futures Forum in Vienna was a similar question: “Has cornea-based refractive surgery peaked?”2 A related question, “Is there life after LASIK?” was prominently featured in a panel discussion at the 2023 AECOS Winter Symposium in Aspen, Colorado.3 The prevalence of such queries in ophthalmic publications, at conferences, and among surgeons highlights growing concerns about the longevity and current state of LVC.
The Refractive Surgery Landscape
From a peak in the United States of approximately 1.2 to 1.4 million procedures annually, LVC volume has declined to around 600,000 to 700,000 procedures today.4 The downturn, noticeable since 2008, has been attributed by some surgeons and economists to the broader economic challenges and a dip in consumer confidence. Nevertheless, the recovery in LVC procedures has not paralleled the general economic rebound from 2012 to 2019. Additionally, despite an initial uptick after the COVID-19 lockdowns, LVC has not experienced the resurgence that was anticipated (Figure).4,5
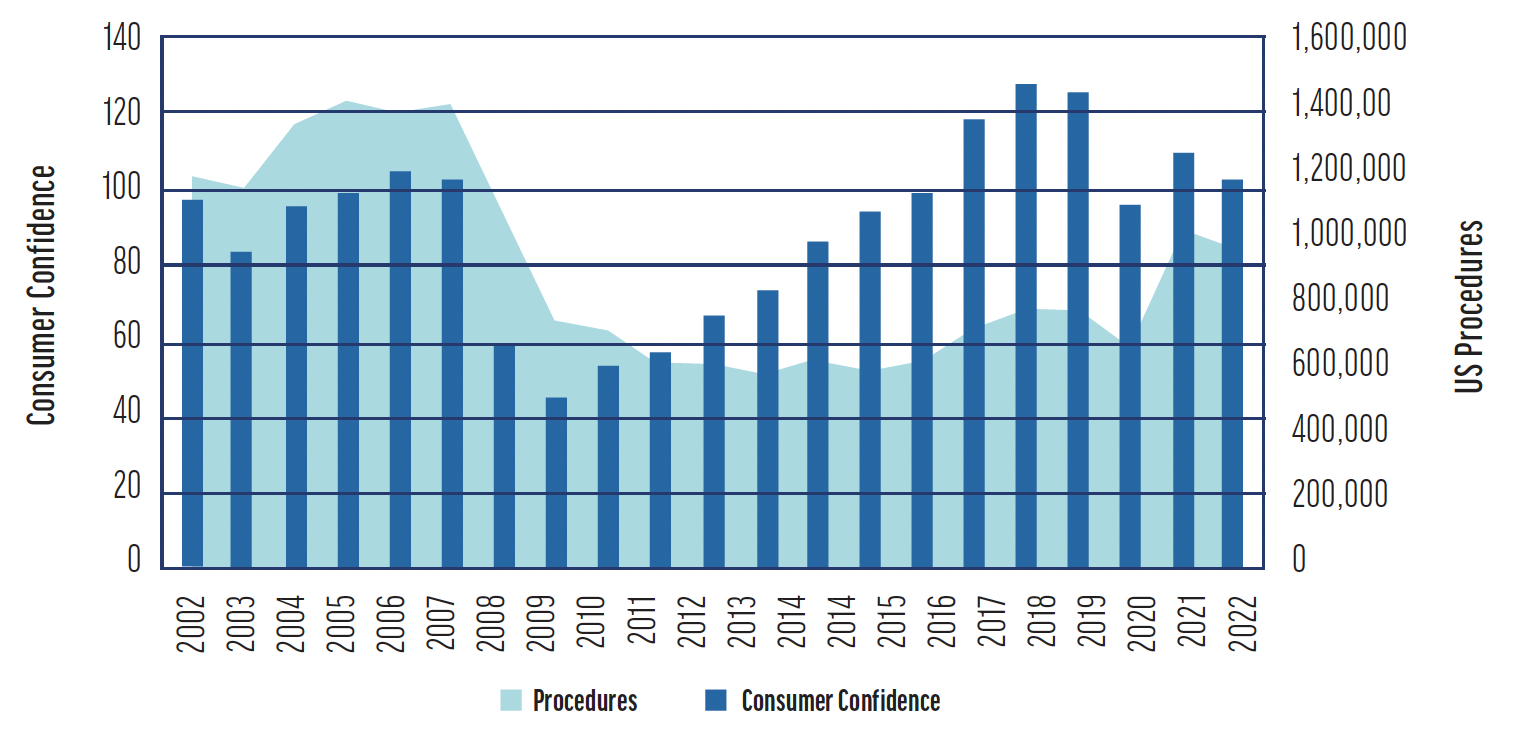
Figure. The correlation between annual US laser refractive surgery volumes and consumer confidence levels from 2002 to 2022 (adapted from The Conference Board, Market Scope, 2022).
Why do the questions posed in the introduction to this article strike me as ominous? The answer lies in their sources. Unlike 15 to 16 years ago, when similar questions came primarily from a small group of dissatisfied patients, the current queries are being raised by refractive surgeons themselves. When the professionals performing LVC question its future, I worry.
A Viable Alternative
When concerns about the future of LASIK and other forms of LVC were raised in 2008, there was no readily available alternative in the United States. Today, those questioning the viability of LVC have an alternative that could offset the decline in LVC or even surpass it as the dominant procedure in refractive surgery: the EVO ICL (STAAR Surgical).
The safety and efficacy of this lens was supported by numerous peer-reviewed studies involving more than 6,000 eyes across several countries.6 In 2022, these lenses received FDA approval following a rigorously conducted trial in the United States. The trial’s results aligned with the positive outcomes documented in international studies during the previous 10 years (Tables 1 and 2).7
Historically, many surgeons considered phakic IOLs only for patients who were not suitable candidates for LVC. In the FDA trial, however, more than one-third of the participants had -3.00 to -6.00 D of myopia and achieved excellent outcomes.8 Before the approval of the EVO ICL, Gregory Parkhurst, MD, and I implanted more than 4,000 Visian ICLs (STAAR Surgical) in soldiers at Fort Hood, Texas, and Fort Bragg, North Carolina. The results were exceptional, particularly with outcome measures of night vision, Snellen acuity, contrast sensitivity, and patient-reported outcomes. Consequently, my colleagues and I began recommending ICLs to soldiers who required the highest-quality visual outcomes, even though they were also suitable candidates for LVC.9-11 Although we appreciated the excellent results achieved with LVC, the ICL was typically chosen in situations where visual quality could mean the difference between success and failure in challenging conditions.
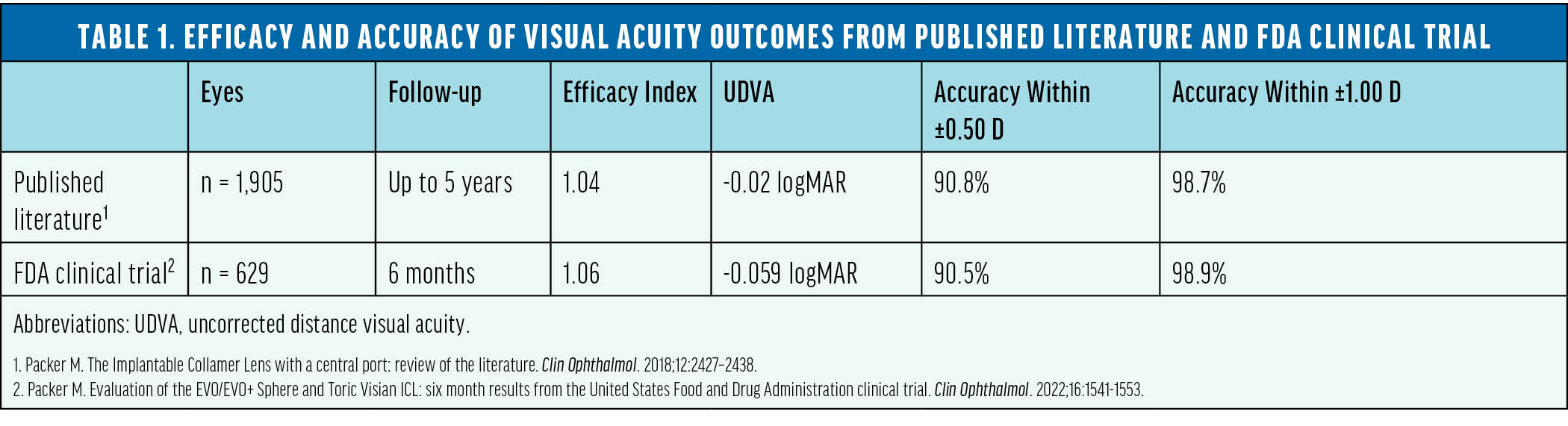
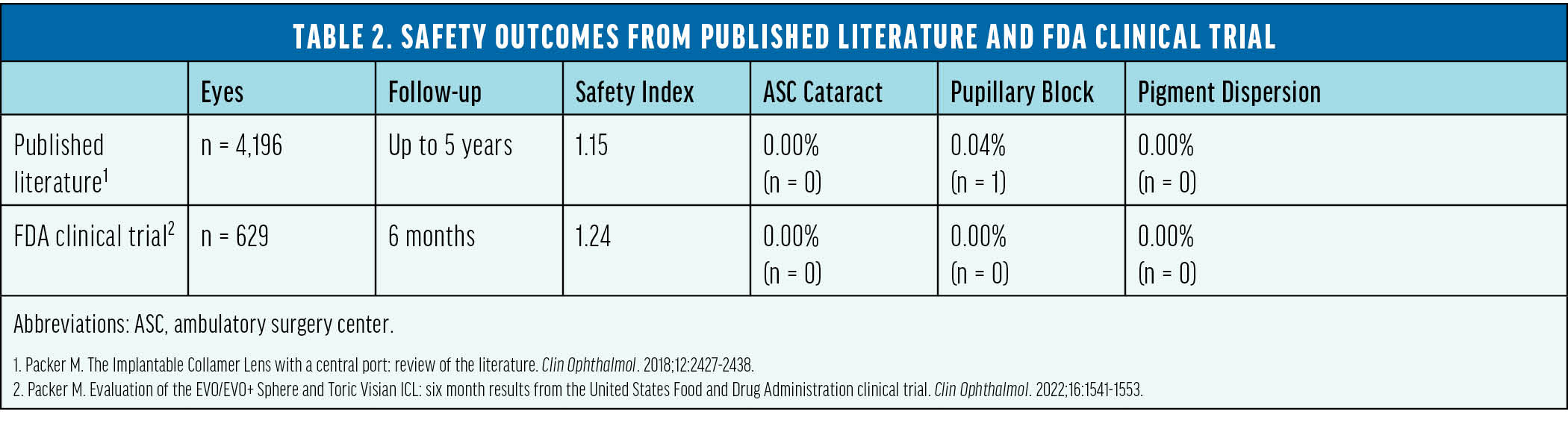
The Popularity of Phakic IOLs
Clinical trials and clinical outcomes have bolstered surgeons’ confidence in offering phakic IOLs as a premium option, even to patients who require a low amount of refractive correction. This trend suggests phakic IOLs could become preferred to LVC. Despite the unparalleled safety and efficacy of the latter, many potential patients remain on the side lines for reasons not fully understood. Declining military LVC volumes indicate that cost is not the sole deterrent.12
The latest advances in phakic IOL technology offer surgeons a viable alternative for these reluctant patients. Shanghai’s pioneering phakic IOL–only clinic (Wow Vision Clinic) established in 2018 and a subsequent one in Tokyo (The Eye Clinic Tokyo Towers) indicate the potential of a practice centered on phakic IOLs. China currently has at least 12 exclusive ICL-only clinics, and surgeons in Japan have developed several other ICL-only clinics. In Japan, public skepticism of LASIK has led to a significant reduction in LVC procedural volume, while phakic IOLs have helped maintain the viability of refractive surgery.13,14 Market Scope analyses have identified a shift from approximately 400,000 refractive procedures annually, before anti-LASIK campaigns, to about 100,000 in 2023, and phakic IOLs account for nearly half of these.5,15,16
The German Commission of Refractive Surgery, following guidance from the German Ophthalmological Society and the Professional Association of German Ophthalmologists, published guidelines for procedural recommendations based on the efficacy and minimal side effects of phakic IOLs.17 After initially endorsing phakic IOLs for corrections starting at -6.00 D, the Commission lowered the threshold to -3.00 D in 2019 and to -1.00 D in 2022 for the Artisan and Artiflex (both from Ophtec) and the EVO ICL. This endorsement, particularly for using low-powered EVO ICLs in eyes typically treated with LVC, demonstrates trust in these lenses.
The Future of Lens-Based Refractive Surgery
The shift toward lens-based refractive surgery is being driven by technological innovation that delivers excellent safety, effectiveness, simplicity (for those skilled in intraocular techniques), and removability. By addressing surgeons’ and patients’ historical concerns, lens-based procedures appear to be poised to offer patients the next level of visual freedom.
1. Is This the End of LASIK? Ophthalmol Times-Europe. September 2023;19(7). Accessed December 12, 2023. https://europe.ophthalmologytimes.com/view/ophthalmology-end-of-lasik-lenticule-extraction-obsolete
2. Has cornea-based refractive surgery peaked? Ophthalmology Futures Forum. Vienna; September 10, 2023. Accessed December 12, 2023. https://www.ophthalmology-futures.com/wp-content/uploads/2023/09/European-Programme-23-Web.pdf
3. Life after LASIK? AECOS 2023 Winter Symposium; February 27, 2023; Aspen, CO. Accessed December 12, 2023. https://aecosurgery.org/wp-content/uploads/2023/02/2023-Aspen-Agenda_Feb24.pdf
4. Lindstrom RL. Millennials will be the next target for laser vision correction. Ocular Surgery News. April 1, 2019. Accessed December 12, 2023. https://www.healio.com/news/ophthalmology/20190329/millennials-will-be-the-next-target-for-laser-vision-correction
5. Market Scope. December 2022.
6. Packer M. The implantable collamer lens with a central port: review of the literature. Clin Ophthalmol. 2018:12:2427-2438.
7. Packer M. Evaluation of the EVO/EVO+ Sphere and Toric Visian ICL: six month results from the United States Food and Drug Administration clinical trial. Clin Ophthalmol. 2022:16:1541-1553.
8. Packer M. The EVO ICL for moderate myopia: results from the US FDA clinical trial. Clin Ophthalmol. 2022;16:3981-3991.
9. Parkhurst GD Psolka M, Kezirian GM. Phakic intraocular lens implantation in United States military warfighters: a retrospective analysis of early clinical outcomes of the Visian ICL. J Refract Surg. 2011;27(7):473-481.
10. Parkhurst GD. A prospective comparison of phakic collamer lenses and wavefront-optimized laser-assisted in situ keratomileusis for correction of myopia. Clin Ophthalmol. 2016:10:1209-1215.
11. Barnes SD. Torics, we don’t need no stinking torics. Presented at: Royal Hawaiian Eye Meeting; January 27, 2017; Maui, HI.
12. Sellers B, Townley JR, Ropp C, Legault G. Refractive surgery trends at tri-service refractive surgery centers and the impact of the COVID-19 pandemic, fiscal years 2000–2020. Med Surveill Monthly Rep. 2022;29(3):18-19.
13. National Consumer Affairs Center of Japan. Do Not Approach Lasik Surgery Lightly, And Be Sure to Receive Full Explanation of the Risks. NCAC News. Vol.25 No.6; March 2014. Accessed December 20, 2023. http://www.kokusen.go.jp/pdf/n-20131204_1.pdf




