
In the words of Sir Harold Ridley, IOLs represent a cure for a disease, and that disease is aphakia. I have had the privilege to work with these fascinating implantable devices since 1999, after finishing a PhD on biomaterials in Paris and starting a fellowship in the IOL pathology laboratory of David J. Apple, MD, at the Medical University of South Carolina, in Charleston. Reflecting on current trends and future directions of IOLs, it is quite evident that they are used with increasing efficiency not only to restore the refractive power of the eye following cataract surgery but also to provide added features and benefits to patients. In this article, I highlight some of the trends in IOL design that are evident since the time of Dr. Apple’s writing for CRST in 2004 (see accompanying excerpt below).
Sir Harold Ridley
A pioneer in the quest to eradicate blindness worldwide.
By David J. Apple, MD
The story of Sir Harold Ridley, born in 1906, and his groundbreaking invention of the IOL is well known and one of the high points of ophthalmology in the 20th century.1-4 In the mid-1980s, many physicians including members of the academic medical establishment still did not accept much of his work.5-8 Even his lens implant,9-11 inarguably one of the most important innovations in the history of ophthalmology and a blessing to society, was widely criticized. Sir Harold’s presentations regarding his invention were often met with skepticism, invective, and scorn. Scientific research on his implant that my colleagues (The Apple Korps) and I had performed in our laboratory between 1982 and 1985 strongly supported and, indeed, verified much of his work.1,2 He was pleased to learn of our publications and, in 1985, summoned me to his retirement cottage in Stapleford, England, near Salisbury, where we first met.4,6 We developed a close personal and professional relationship that lasted until his death on May 25, 2001.
POLITICS IN OPHTHALMOLOGY
Overview. Contributing to the lack of recognition for Sir Harold was the fact that some clinical complications occurred early on, many due to variations in the surgical techniques of other surgeons. These complications made some ophthalmologists hesitant to implant an IOL. Also, Sir Harold was not an aggressive advocate of lens implantation. My research then and as late as 2 years after his death3,4 has clearly demonstrated that numerous vagaries of ophthalmic politics (ie, jealousy and a lack of foresight) were the major causes of our specialty’s long-delayed acceptance of IOLs.5-8
Ridley versus Duke-Elder. It is no secret that Sir Harold had major differences with one of the most prominent ophthalmologists of the 20th century, Sir Stewart Duke-Elder. They had various disagreements as early as the 1930s, at which time Sir Harold was the youngest ophthalmologist to receive a full consultant appointment at Moorfields Eye Hospital, in London. Their differences increased as World War II began.
Even after the war, following Sir Harold’s invention of the IOL,9-13 he and Sir Stewart could not communicate. The latter was never able to accept the IOL.
RESISTANCE TO THE IOL
The huge backlash against Sir Harold’s invention and surgical procedure (Figure) by many of his peers occurred within the academic establishment in both England and the United States.

Figure. Sir Haroldʼs cinematographer-filmed IOL operation No. 7 in May 1951 (left). Sir Harold has grasped the IOL (Rayner) for insertion into the open wound, immediately after removing the cataractous, opaque crystalline lens. The operation was performed with minimal draping. Most surgeons of that era, including Sir Harold, wore no gloves, and lighting was provided by the simple flashlight held by the nurse. The IOL was a round, plastic disc (right).
Based on numerous interviews with his contemporaries who observed the evolution of the IOL firsthand, the emergence of this device necessitated a radical paradigm shift. Prior to that time, eye surgeons routinely learned to take things out of the eye (ie, foreign bodies, inflammatory material, tumors). The implementation of the IOL required a new thought pattern, namely, the concept of putting something into the eye. This idea was difficult for many to accept. Sir Harold’s IOL9-13 was a new and important adjunct to cataract surgery (in his own words, a “cure of aphakia”), but it also represented a much broader and more significant innovation. Without realizing it, Sir Harold effectively helped to pioneer the modern field of biomedical engineering, specifically, the field of artificial device implantation. His IOL long preceded the introduction of all major devices designed for tissue/organ replacement and implantation into the body’s various delicate and vital tissues and organs (eg, heart pacemakers, artificial kidneys, artificial hearts).
ENTERING THE MODERN ERA
During the latter half of the past century, the field of ophthalmology sufficiently recovered from the wreckage of World War II to progress into the era that we now term the Golden Age of Ophthalmology and the Visual Sciences. Sir Harold’s inventions represented an actual and symbolic beginning of this modern period, characterized by (1) extensive closures of institutes and sanitaria for the blind, (2) the substantial decrease in the number of blinded eyes submitted to anatomic-pathology laboratories such as the one directed by myself since the 1970s, and (3) surgeons’ markedly increased ability to treat and manage eyes injured by various types of disease or trauma—eyes almost assuredly lost in former times.
ACCEPTANCE
One pivotal event that helped remedy Sir Harold’s lack of acceptance occurred in 1986, when he was elected a fellow of the highly prestigious Royal Society. Further, in 1989, I presented his credentials and a list of his accomplishments to my former university, the Medical University of South Carolina. I was dumbfounded that some members of the administration and even my department were loath to honor Sir Harold because they were unaware of his scientific and clinical accomplishments and of his gift to humanity. Fortunately, the president of the university at that time, Dr. James B. Edwards, rapidly grasped my intentions, and the university did agree to confer an honorary doctor’s degree on Sir Harold that year.
A torrent of honors for him followed, and he was belatedly knighted in London by Queen Elizabeth II on February 9, 2000, less than 1 year before his death.
1. Apple DJ, Kincaid MC, Mamalis N, et al. Intraocular Lenses: Evolution, Designs, Complications, and Pathology. Baltimore, MD: Williams and Wilkins; 1989.
2. Apple DJ, Mamalis N, Googe JM, et al. Complications of intraocular lenses. A histopathological review. Surv Ophthalmol. 1984;29:11-54.
3. Apple DJ, Ram J, Foster A, Peng Q. Elimination of cataract blindness. A global perspective entering the new millennium. Surv Ophthalmol. 2000;45(special delivery suppl):1-196.
4. Apple DJ. Auffarth GU, Peng Q, Visessook N. Foldable Intraocular Lenses. Evolution, Clinicopathologic Correlation, and Complications. Thorofare, NJ: Slack; 2000.
5. Theobald GD. In regard to the Ridley implant. Arch Ophthalmol. 1953:36:1471.
6. Duke-Elder S. System of Ophthalmology: Diseases of the Lens and Vitreous, Glaucoma and Hypotony. Vol. XI. St. Louis, MO: CV Mosby; 1969:1-291.
7. Vail D. Discussion of Ridley, H. Further observations on intraocular acrylic lenses. Trans Am Acad Ophthalmol Otolaryngol. 1953;57:104:99.
8. Vail D. A dream cometh through a multitude of business. Am J Ophthalmol. 1952;35:1701-1703.
9. Ridley NHL. Intraocular acrylic lenses. Trans Ophthalmol Soc UK & Oxford Ophthalmol Congress. 1951:LXXI:617-621.
10. Ridley NHL. Intraocular acrylic lenses after cataract extraction. Lancet. 1952;1: 118-119.
11. Ridley NHL. Further observations on intraocular acrylic lenses in cataract surgery. Trans Am Acad Ophthalmol Otolaryngol. 1953;57:98-106.
12. Choyce DD. Intra-Ocular Lenses and Implants. London, England: H. K. Lewis & Co; 1964.
13. Apple DJ, Sims J. Harold Ridley and the invention of the intraocular lens. Surv Ophthalmol. 1996;40:279-292.
OVERALL DESIGN, MATERIALs
In general, more IOLs are now available in preloaded disposable delivery systems, thus saving time, allowing more consistent delivery, eliminating IOL touch, and potentially increasing safety.1 Surgeons continue to prefer one-piece IOL designs rather than multipiece lenses, which in some practices are mostly reserved for cases in which in-the-bag implantation is jeopardized.
Hydrophobic acrylic IOL materials appear to have the largest share of the market worldwide. Because a potential concern with this class of materials is the presence of hydration-related inhomogeneities such as glistenings, recent years have seen the increasing development and availability of hydrophobic acrylic lenses with water content higher than the previous standard of less than 0.5%.2,3 These lenses still meet the criteria for this class of materials, exhibiting hydrophobic characteristics, but they are considered glistenings-free.
Aspheric IOLs, generally designed to neutralize positive spherical aberration in the eye, continue to be a trend in IOL manufacturing, although approaches to asphericity differ among manufacturers. Other continuing trends include the incorporation of a blue-light filter into the IOL material, in addition to the standard UV-light protection, and the manufacture of IOLs for insertion through small incisions, generally 1.8 to 2.2 mm.
PRESBYOPIA CORRECTION
There has been continued interest in the development of presbyopia-correcting IOLs. New bifocal and trifocal IOLs are continually introduced to the market (Figure 1), the latter more so in Europe than in the United States, including lenses with concomitant toric correction.4,5

Figure 1. New bifocal and trifocal IOLs, such as the FineVision trifocal (PhysIOL), are continually introduced to the market today.
Another trend in this area is the concept of extended depth of focus (EDOF) IOLs.5,6 These lenses aim to produce a wide focal point with an increased depth of focus, unlike traditional multifocal IOLs, which generate two or more separate focal points for defined viewing distances. EDOF IOLs potentially have less negative impact on quality of vision than traditional multifocal lenses, and they place less demand on the surgeon to achieve emmetropia. Different EDOF designs enhance depth of focus with different mechanisms, including use of achromatic IOL technology, zones with different aspheric profiles, and the pinhole effect (Figure 2).7

Figure 2. EDOF lenses use different mechanisms to increase depth of focus, such as the pinhole effect in the IC-8 (AcuFocus).
OTHER TRENDS
Supplementary or add-on IOLs, specifically designed to be fixated in the ciliary sulcus in the pseudophakic eye, are now being developed to provide more than just the possibility to correct residual refractive errors. Certain models can also provide multifocality, toric correction, a pinhole effect, and magnification for patients with age-related macular degeneration (AMD).8-10
Toric IOLs have been available for many years, but the pinhole principle has more recently been incorporated into IOLs for the management of irregular astigmatism. Small-aperture IOLs, designed as add-on lenses for sulcus fixation in patients already pseudophakic (Figure 3) or for primary in-the-bag implantation (Figure 2), are now available.7-9 The pinhole effect can also provide an enhanced depth of focus, as mentioned previously.

Figure 3. Small-aperture IOLs, such as the XtraFocus (Morcher), have also been designed as add-on lenses for sulcus fixation in patients who are already pseudophakic.
Relatively bulky implantable telescope IOLs have been available for patients with end-stage AMD for some time, but foldable IOLs with more standard designs that can be inserted through small incisions have more recently been developed. These lenses have been demonstrated to enhance quality of life and independence in daily activities for patients with advanced AMD. There are lenses with an optic area that provides high add power now available for primary in-the-bag implantation, as well as add-on lenses for sulcus fixation in patients who are already pseudophakic (Figure 4).10

Figure 4. Foldable lenses with high add powers, such as the Scharioth Macular Lens (Medicontour), have been designed as add-ons for sulcus fixation.
DESIGNS FOR LASER SURGERY
Surgical techniques can have an impact on the development of new IOLs. The advent of laser cataract surgery, with its ability to create consistent capsular openings, renewed the interest in capsulotomy-fixated IOLs, which potentially offer benefits in terms of predictability and stability of IOL centration and tilt, control of capsular phimosis, elimination of negative dysphotopsia, and rotational and refractive predictability and stability. A new IOL designed to be used in conjunction with laser cataract surgery has a haptic system with two large and two small flaps that allow the lens to clamp into the capsulotomy (Figure 5).11
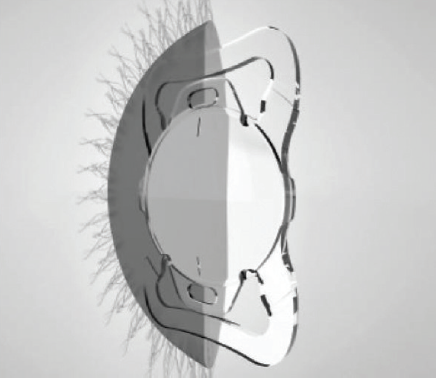
Figure 5. The Femtis IOL (Oculentis) was designed to be used in conjunction with laser cataract surgery, employing a haptic system with two large and two small flaps that allow the lens to clamp into the capsulotomy.
Grooved, capsulotomy-centered IOLs were available long before the use of laser cataract surgery became widespread. These IOLs prevent posterior capsular opacification because anterior and posterior capsulotomies are fitted into the groove in the lens periphery (ie, bag-in-the-lens technique).12 More recently designed antidysphotoptic IOLs have an annular groove on the peripheral portion of the anterior surface of the optic to receive the anterior capsulotomy, while the rest of the lens remains within the capsular bag.13
ADJUSTABILITY
The concept of the adjustable IOL continues to be a hot topic. IOLs designed as modular systems permit easier and safer surgical exchange of the optic component only, allowing surgeons to deal with residual refractive errors or postoperative anatomic changes after cataract surgery (Figure 6).14 Such systems also, in theory, grant the patient access to new optical technologies yet to be developed.

Figure 6. The Harmoni Modular IOL System (ClarVista Medical) permits surgical exchange of the optic component only, allowing surgeons to address residual refractive errors after cataract surgery.
Lenses whose power can be adjusted by light application postoperatively in a noninvasive manner are already available in some markets. A drawback may be the fact that use of UV-protective spectacles is necessary until the final power of the IOL is locked in, after which no more adjustments are possible.15
Great progress has been made in research regarding use of femtosecond laser technology to create refractive index changes in targeted areas of implanted IOLs.16,17 If fully realized, this technology will work on IOLs already on the market—not just one new specific type of lens. Clinical evaluation of noninvasive IOL power adjustment that can be accomplished through this technology is about to begin.
The quest also continues for the truly accommodating IOL, and projects that rely more on shape-related changes in the surfaces of the lens, including modular and nonmodular fluid-filled lenses, are being evaluated in clinical trials (Figure 7).18

Figure 7. Fluid-filled lenses, including the FluidVision (PowerVision), are being evaluated in clinical trials.
CONCLUSION
With many years’ worth of innovative ideas and IOL designs already in the market, I anticipate that we in the ophthalmologic community will continue to marvel at what can be accomplished through implantation of these tiny devices for many years to come.
1. Wang L, Wolfe P, Chernosky A, Paliwal S, Tjia K, Lane S. In vitro delivery performance assessment of a new preloaded intraocular lens delivery system. J Cataract Refract Surg. 2016;42(12):1814-1820.
2. Packer M, Williams JI, Feinerman G, Hope RS. Prospective multicenter clinical trial to evaluate the safety and effectiveness of a new glistening-free one-piece acrylic toric intraocular lens. Clin Ophthalmol. 2018;12:1031-1039.
3. Werner L, Ellis N, Heczko JB, et al. In vivo evaluation of a new hydrophobic acrylic intraocular lens in the rabbit model [published online ahead of print September 15, 2018]. J Cataract Refract Surg.
4. Steinwender G, Schwarz L, Böhm M, et al. Visual results after implantation of a trifocal intraocular lens in high myopes. J Cataract Refract Surg. 2018;44(6):680-685.
5. Cochener B, Boutillier G, Lamard M, Auberger-Zagnoli C. A comparative evaluation of a new generation of diffractive trifocal and extended depth of focus intraocular lenses. J Refract Surg. 2018;34(8):507-514.
6. Savini G, Balducci N, Carbonara C, et al. Functional assessment of a new extended depth-of-focus intraocular lens [published online ahead of print September 28, 2018]. Eye (Lond).
7. Dick HB, Elling M, Schultz T. Binocular and monocular implantation of small-aperture intraocular lenses in cataract surgery. J Refract Surg. 2018;34(9):629-631.
8. Trindade CC, Trindade BC, Trindade FC, Werner L, Osher R, Santhiago MR. New pinhole sulcus implant for the correction of irregular corneal astigmatism. J Cataract Refract Surg. 2017;43(10):1297-1306.
9. Tsaousis KT, Werner L, Trindade CLC, Guan J, Li J, Reiter N. Assessment of a novel pinhole supplementary implant for sulcus fixation in pseudophakic cadaver eyes. Eye (Lond). 2018;32(3):637-645.
10. Scharioth GB. New add-on intraocular lens for patients with age-related macular degeneration. J Cataract Refract Surg. 2015;41(8):1559-1563.
11. Schojai M, Schultz T, Burkhard Dick H. Capsule-fixated intraocular lens implantation in small pupil cases. J Refract Surg. 2017;33(8):568-570.
12. Tassignon MJ, De Groot V, Vrensen GF. Bag-in-the-lens implantation of intraocular lenses. J Cataract Refract Surg. 2002;28(7):1182-1188.
13. Masket S, Fram NR, Cho A, Park I, Pham D. Surgical management of negative dysphotopsia. J Cataract Refract Surg. 2018;44(1):6-16.
14. Guan JJ, Kramer GD, MacLean K, et al. Optic replacement in a novel modular intraocular lens system. Clin Exp Ophthalmol. 2016;44(9):817-823.
15. Hengerer FH, Müller M, Dick HB, Conrad-Hengerer I. Clinical evaluation of macular thickness changes in cataract surgery using a light-adjustable intraocular lens. J Refract Surg. 2016;32(4):250-254.
16. Sahler R, Bille JF, Enright S, Chhoeung S, Chan K. Creation of a refractive lens within an existing intraocular lens using a femtosecond laser. J Cataract Refract Surg. 2016;42(8):1207-1215.
17. Werner L, Ludlow J, Nguyen J, et al. Biocompatibility of intraocular lens power adjustment using a femtosecond laser in a rabbit model. J Cataract Refract Surg. 2017;43(8):1100-1106.
18. Kohl JC, Werner L, Ford JR, et al. Long-term uveal and capsular biocompatibility of a new accommodating intraocular lens. J Cataract Refract Surg. 2014;40(12):2113-2119.




