CASE PRESENTATION
A 56-year-old woman is referred for a cataract evaluation. The patient has had nystagmus and amblyopia since childhood. The severity of the amblyopia is unknown because she does not recall what her BCVA was before her cataracts developed. She underwent implantation of a Verisyse IOL (Johnson & Johnson Vision) in each eye to treat high myopia in 2005. Her medical history is notable only for asthma. The patient is a mental health therapist who enjoys painting, reading, and crafting in her free time.
On presentation, her UCVA is 20/50+2 and J3- OD and 20/300 and J3- OS. Her BCVA is 20/40-2 OD with a manifest refraction of -0.25 D sphere and J2 with an add of +2.00 D. Her BCVA is 20/60-2 OS with a manifest refraction of -4.00 -0.75 x 115º and J2 with an add of +2.00 D. On glare testing, the patient’s vision is light perception with this prescription. Figures 1 to 4 show her preoperative measurements.
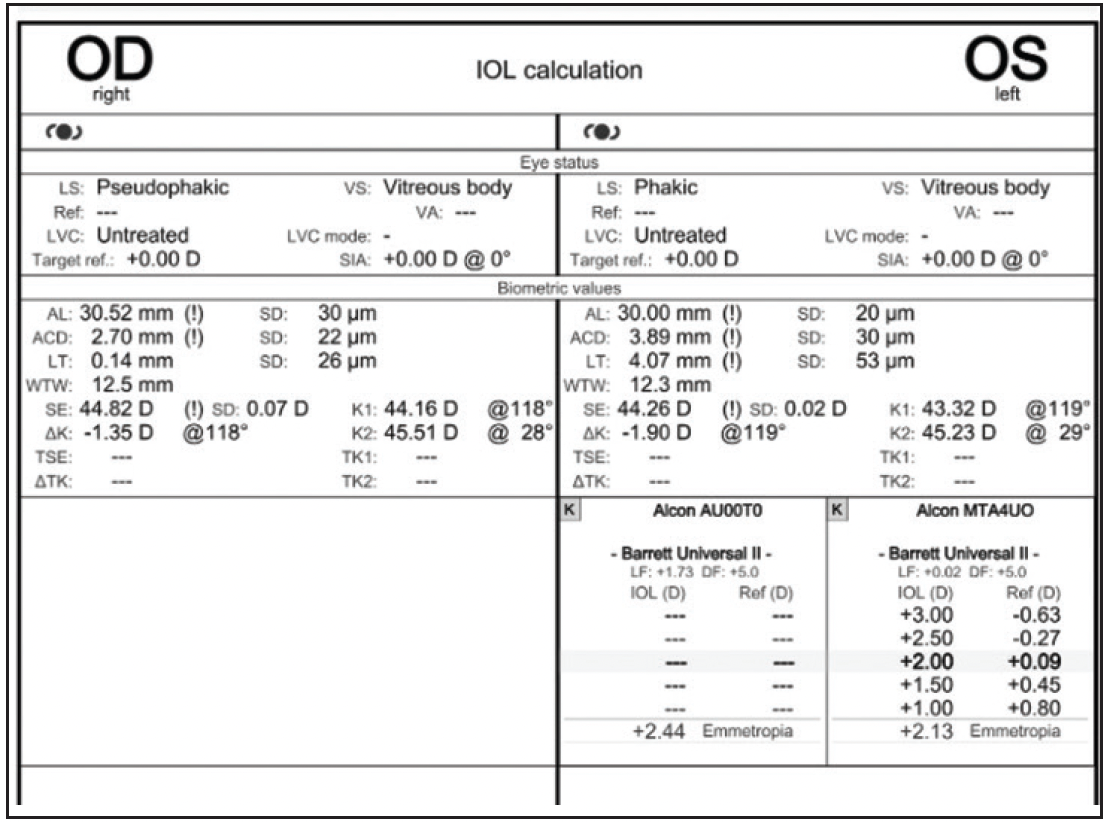
Figure 1. Measurements with the IOLMaster (Carl Zeiss Meditec).
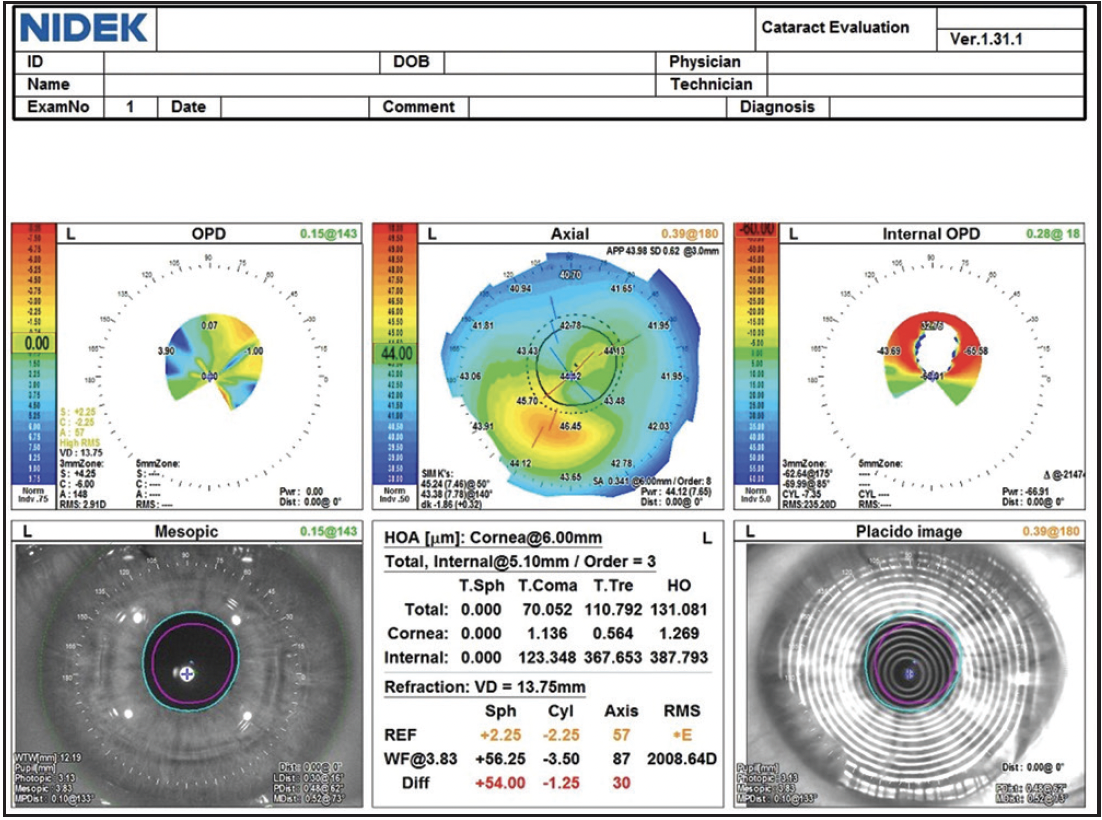
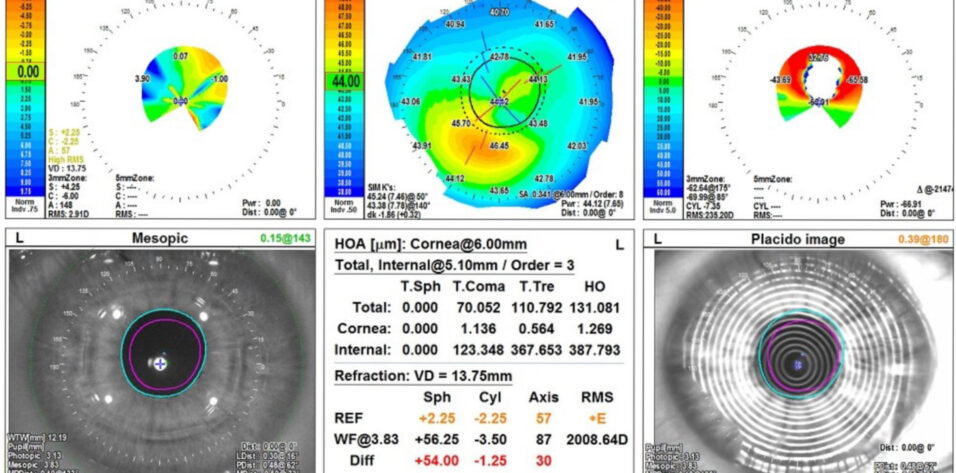
Figure 2. Measurements of the left eye with the OPD-Scan (Nidek).
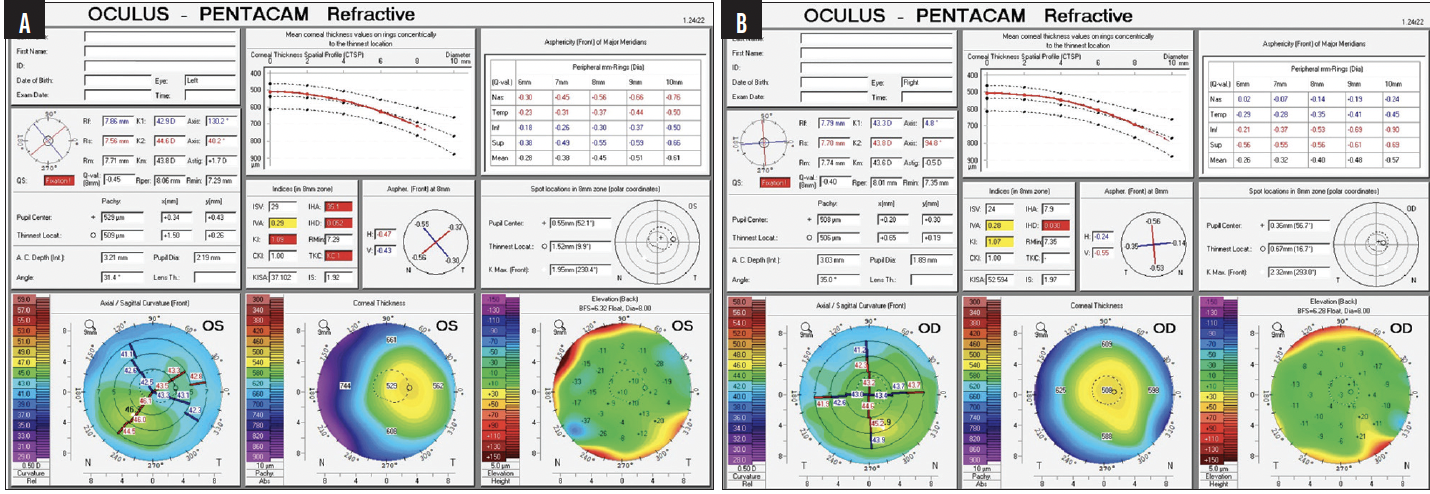
Figure 3. Measurements of the left (A) and right (B) eyes with the Pentacam (Oculus Optikgeräte).
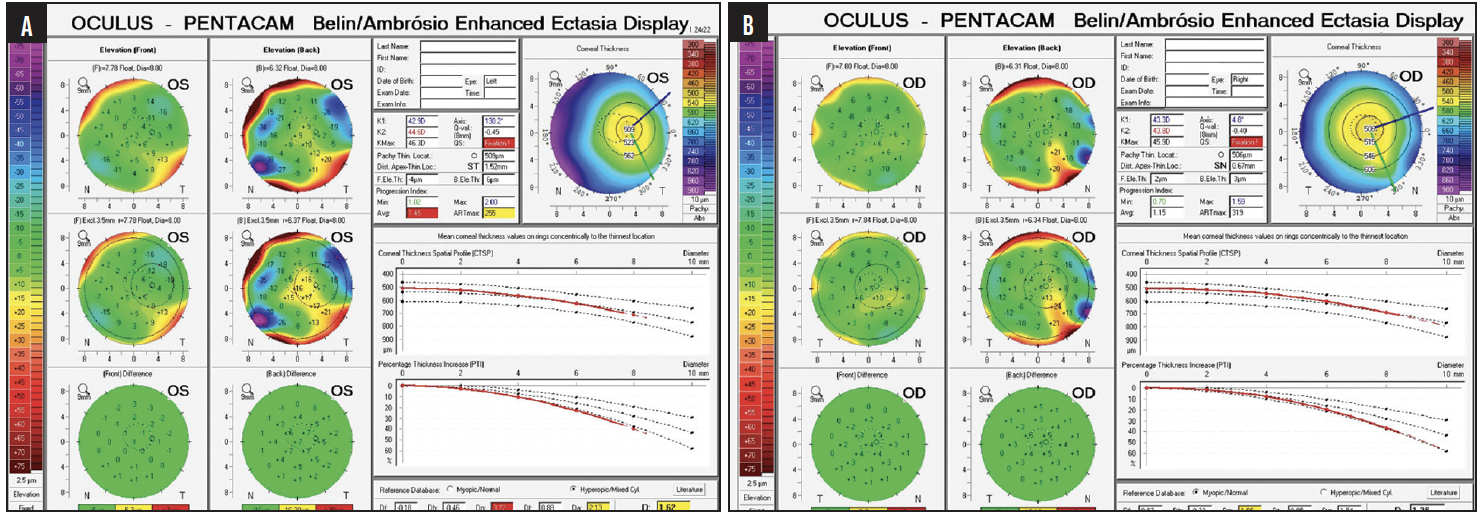
Figure 4. Ectasia risk assessment of the left (A) and right (B) eyes.
An iris-clipped phakic IOL is visible in each eye at the slit lamp. The right eye has 2+ nuclear sclerosis and a trace cortical cataract. The left eye has 3+ nuclear sclerosis and a trace posterior subcapsular cataract. Nystagmus makes examining the fundus difficult. Fleeting glances suggest a grossly normal fundus and chorioretinal scar in the periphery of the right eye. The size of the dilated pupil is 6.9 mm OU.
The patient reports that a plano result was originally targeted in each eye. She does not recall having tried monovision in the past and until now was unaware of her current monovision state from a myopic shift in the left eye.
She is extremely anxious about undergoing surgery but can no longer function and feels unsafe driving. She describes receiving what sounds like a lid block and general anesthesia for surgery in 2005—presumably owing to her large and rapid nystagmus. She inquires about general anesthesia for cataract surgery, but after a discussion of the risks and benefits, she is open to local anesthesia with monitored anesthesia care.
The patient is intrigued by the possibility of postoperative spectacle independence, but it is not a high priority for her. Her main objective is to feel more confident working and driving again. She wants to know all her options, however, and which would be the best for her. Air travel is required for all office visits. She prefers not to make frequent trips or spend a large amount of money unless necessary, but she is willing to assume some out-of-pocket expenses to achieve a medical benefit.
How would you proceed?
—Case prepared by Neda Nikpoor, MD

P. DEE G. STEPHENSON, MD, FACS
I would begin with an extensive discussion of the patient’s situation and the risks and benefits of surgery. Several face-to-face conversations would be held throughout her journey to reduce her stress level as much as possible. My goal would be to underpromise and overdeliver on results.
I find that general anesthesia minimizes patient and surgeon anxiety in situations like this one. Forms of anesthesia that cause muscle contraction, such as ketamine, would be avoided. The patient would undergo pretreatment with antinausea medication to reduce her risk of vomiting after surgery.
Laser cataract surgery with the Ally Adaptive Cataract Treatment System (Lensar) would be a good option because of the patient’s nystagmus. I have found this platform to be extremely fast and accurate. The phakic IOLs could be removed first because I am not sure if femtosecond laser energy can pass through them. Whether the pupil size is adequate for laser cataract surgery is another consideration. After removal of the phakic IOL, the eye would be reinflated with balanced salt solution. The patient would then be positioned under the femtosecond laser, the cataract and astigmatic incisions created, and the nucleus presoftened. All that said, traditional cataract surgery would probably be a safer approach.
An effort would be made to identify the null point at which the patient’s nystagmus is minimal, and A-scan ultrasound biometry would be repeated multiple times until a somewhat consistent scan is obtained. The same approach would be taken with topography and, if possible, analysis with the iTrace (Tracey Technologies).
Phakic IOL removal would be combined with cataract surgery and IOL implantation. Phenylephrine 1% and ketorolac 0.3% (Omidria, Rayner) would be administered intraoperatively for mydriasis and control of pain and inflammation. My preference would be to implant an aspheric IOL that has the same power from its center to its edge because the asphericity can benefit the patient even if the null point does not align with the center of the pupil. The enVista IOL platform (Bausch + Lomb) fulfills these criteria and is also a good choice for someone with amblyopia. The patient is not a candidate for a multifocal or small-aperture lens because of her nystagmus and pupillary axis.
Unfortunately, the use of general anesthesia precludes the use of intraoperative aberrometry.

GARY WÖRTZ, MD
Surgery is likely to be challenging and would therefore be performed under general anesthesia. The patient’s large eyes, moreover, increase the risk of reverse pupillary block, and the associated pain could cause inadvertent movement. This would make suturing the 6-mm corneal incision required to remove the phakic IOL more difficult.
Given the risk of retinal detachment in highly myopic eyes and the possible future need for silicone oil, I would favor an acrylic monofocal or toric IOL, preferably one that is neutrally aspheric. The risk of suction loss due to nystagmus precludes the use of a femtosecond laser.
Because the patient must fly in for all appointments and general anesthesia is advisable, immediately sequential bilateral cataract surgery would be combined with phakic IOL removal. If possible, the phakic lens would be removed through a separate incision, located perhaps superiorly and subsequently sutured closed. Phacoemulsification would then be performed through a temporal incision.
Her history of amblyopia, among other issues, would prevent me from recommending any refractive target other than bilateral distance vision. The patient’s desire for spectacle independence would not play much of a role in my decision. Functional vision with glasses is the goal. Anything better would be a welcome bonus.
Considering both the astigmatism that will be induced by the large incisions required for surgery and the patient’s inability to fixate preoperatively, intraoperative aberrometry could be highly beneficial.

WHAT I DID: NEDA NIKPOOR, MD
As the panelists explain beautifully, this case presented several challenges. I largely agree with the excellent approaches they propose, but how I proceeded in this situation differed somewhat.
I chose to perform sequential surgery. First, each phakic IOL was removed through a superior, self-sealing, sclerocorneal tunnel incision similar to that made during manual small-incision cataract surgery. Three months later, the cataract procedure was performed. The reasons for my decision were (1) improved chamber stability and fluid dynamics due to the large, fully sealed incision and (2) greater refractive predictability. I was concerned that a large scleral tunnel might alter the patient’s biometry significantly and thought that, if she were willing to come in for four total surgeries (two per eye) spaced out over many months, the chances of hitting the refractive target would be higher. The patient recognized the value of both potentially greater intraoperative safety and a possibly better refractive outcome and agreed to the proposed strategy.
Another consideration was that I do not have access to general anesthesia at my center. When we discussed a possible referral to another provider who could offer general anesthesia, the patient stated that she preferred to stay in my care because of the rapport and trust we had established. Moreover, after a thorough discussion of her concerns and fears, she decided she would not require general anesthesia. I strongly agree with Dr. Stephenson’s point that lengthy, face-to-face conversations with the patient are crucial in situations like this one. These talks can reduce patients’ anxiety, which usually improves their experience and outcomes.
Midazolam was administered intravenously during the first procedure. Interestingly, the anesthesia dramatically attenuated the nystagmus. When I shared this observation with the patient, she stated that her nystagmus worsens when she is anxious and lessens when she is calm. Based on this experience, I feel it may be worth asking patients with nystagmus whether they notice dampening or worsening of their nystagmus in certain situations if topical and monitored anesthesia care is planned.
I decided to perform laser cataract surgery. Because of the general rapidity of treatment with the Ally Adaptive Cataract Treatment System in my experience, I was not overly concerned about the possibility of suction loss. In my experience, the laser capsulotomy, fragmentation, arcuate incisions, and clear corneal incisions take 1, 5 to 7, 2, and 6 seconds, respectively. Although the capsulotomy could have been performed manually, I felt that the precision and control offered by the laser system could offer greater safety and predictability.
The eye was stabilized during all steps of the cataract procedure with a 0.12 forceps or a second instrument. Although I do not perform bimanual irrigation and aspiration routinely, I switched to bimanual mode on the Intrepid Transformer I/A handpiece (Alcon) whenever the nystagmus seemed to be causing sudden eye movement. Phenylephrine 1% and ketorolac 0.3% were administered intraoperatively to help maximize success in this difficult case.
With regard to refractive outcomes, I counseled the patient to have low expectations. Because of her amblyopia, she decided a toric IOL was not worth the cost. Instead, a monofocal IOL with zero spherical aberrations (enVista) was implanted. Conservative laser arcuate incisions were made to reduce astigmatism. Given the patient’s high amount of axial myopia and recommended IOL powers of 3.00 D OD and 2.00 D OS, intraoperative aberrometry with the ORA System (Alcon) was performed, and 2.00 and 3.00 D IOLs were implanted in the right and left eyes, respectively. Although the keratometry readings changed after phakic IOL removal, the impact was minimal because a nontoric monofocal IOL was implanted. The changes in IOL power and in the axis and magnitude of astigmatism nevertheless illustrate the value of a staged approach to surgery in situations like this one.
The patient was pleased with her outcome and felt it was worth the multiple visits. At her most recent visit with the referring physician, her UCVA was 20/50 OD, which did not improve with pinhole testing, and 20/30 OS. The right eye was being treated for mild cystoid macular edema.