Managing dry eye symptoms is part of any ophthalmologist’s practice, regardless of their subspecialty. In an increasingly technology-driven world, the prevalence of situational dry eye, triggered by prolonged screen exposure, shows no signs of abating. In this month’s “Fundamentals in Five,” we are pleased to present an article from Dr. Sangwan and coauthors. Their comprehensive guide delves into the classification and systematic treatment of dry eye disease, with a special focus on issues related to digital device usage.
Kavitha R. Sivaraman, MD
Section Editor




Dry eye disease (DED) typically presents in one of three forms: aqueous-deficient, evaporative, or mixed. Differentiating among them can be challenging, given the overlap of clinical symptoms between aqueous-deficient and evaporative DED.1
Tear film instability, epithelial damage, and clinically evident inflammation all contribute to DED.2 Regardless of the underlying cause, DED perpetuates a cycle involving lipid layer reduction, tear film instability, and increased tear evaporation.3
1. PREVALENCE OF DED
Evaporative DED is the most common form of the disease encountered in clinical practice, making it the dominant subtype.4 The condition often results from eyelid-related issues, such as abnormal blinking or meibomian gland dysfunction (MGD), and/or ocular surface problems, such as mucin deficiency.2,5
An increase in the use of digital devices, including smartphones, computers, and tablets, contributes to the prevalence of evaporative DED,6 which ranges from 9.5% to 87.5% among office workers who use computers and similar screens extensively.3,7 MGD is the primary cause of evaporative DED.8 The impact, however, of altered blinking patterns and blink frequency on tear film stability in the context of evaporative DED merits further research.
2. IMPACT OF BLINKING MECHANISM ON EVAPORATIVE DED
Blinking helps to maintain ocular surface health, ensure tear film stability, distribute lipids across the tear film, and facilitate tear drainage.9,10 Each blink distributes lipids from the lower meibomian glands over the aqueous tear film, forming its lipid layer. Thinning of this layer is influenced by surface tension (Figure 1).11
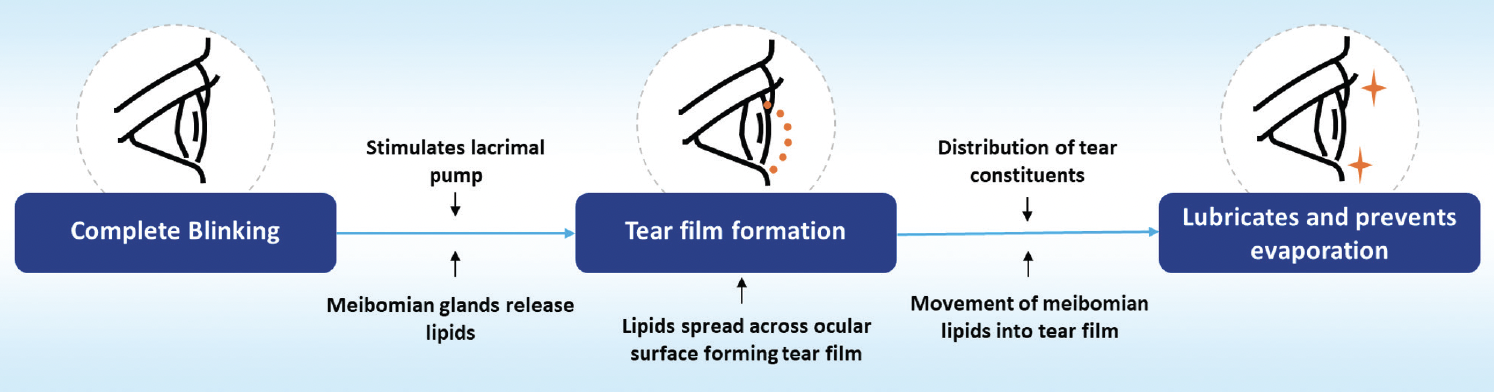
Figure 1. The role of blinking in tear film dynamics.9,11,13
Blinking patterns are activity dependent. A person’s blink rate is approximately 4.5 times per minute while reading, 17 blinks per minute in a silent primary gaze, and 26 blinks per minute when engaged in conversation.12 Alterations in blinking patterns2 and an increased palpebral aperture during digital device use are strongly associated with DED development.12-14
The intensive visual processing and cognitive load during digital device use often lead to a decreased blink frequency or incomplete blinks and contribute to evaporative DED (Figure 2).15
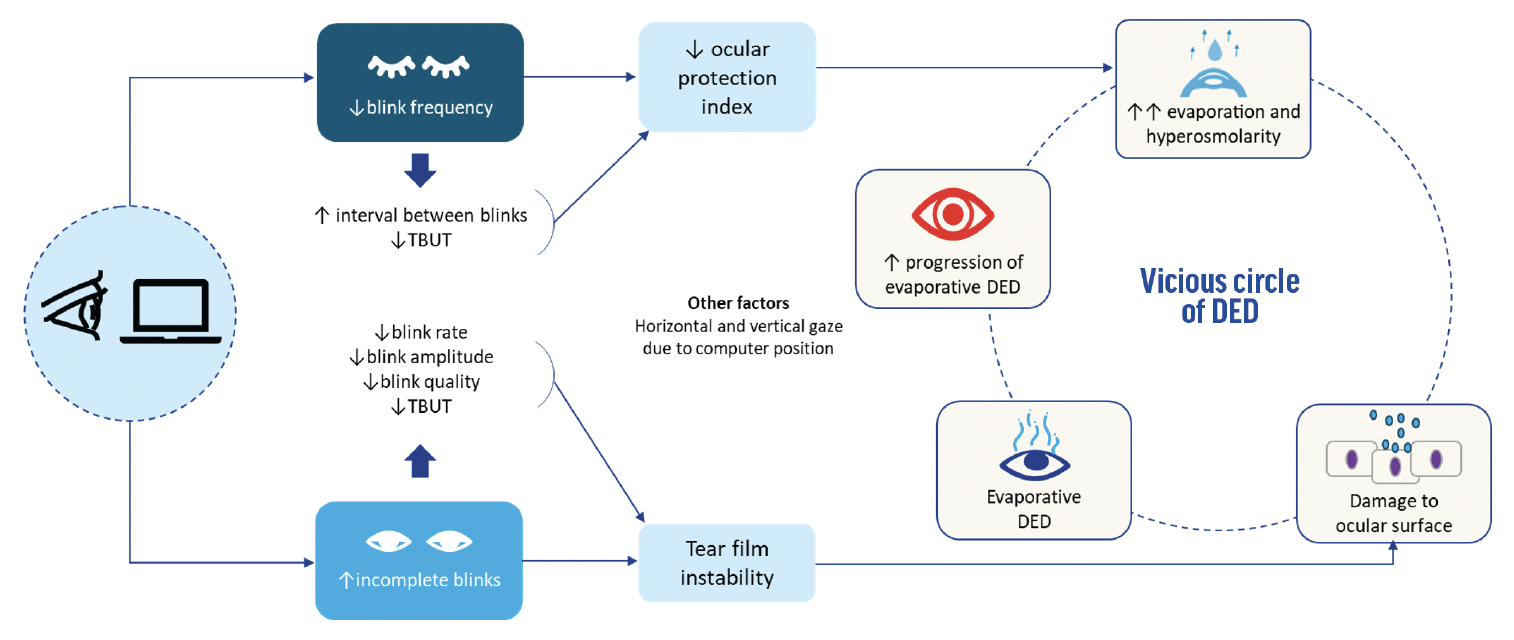
Figure 2. The blinking mechanism in digital screen users and DED.3,6,15,17
Incomplete blinks, reduced blink rate, blink amplitude, and blink quality. Digital screen users often exhibit incomplete blinking, characterized by a reduced blink rate, amplitude, and quality. This can result in inadequate distribution of meibomian lipids to the tear film.13
Reduced blink frequency. A decrease of around 40% to 60% in the spontaneous blink rate has been observed during computer use.10 This decline, along with increased tear evaporation, extends the interval between blinks and shortens the tear film breakup time (TBUT),6,13,16,17 lowering the ocular protection index score.3
Other influences. The typical positioning of digital screens can encourage a horizontal or upward gaze, which increases the palpebral aperture and exposes more of the ocular surface to evaporation.18
The disruption of the lipid layer caused by altered blinking patterns during digital device use can leave the ocular surface unprotected for long periods. This, in turn, can increase tear evaporation and cause hyperosmolarity, perpetuating the vicious circle of DED.3
3. DIAGNOSING EVAPORATIVE DED
The absence of a universally accepted gold standard test can make diagnosing evaporative DED challenging.2 Corneal/conjunctival surface staining, the Schirmer test, interferometry, and OCT measurements of tear volume, TBUT, and tear meniscus height19 can differentiate between evaporative and aqueous-deficient DED. The widespread implementation of these tests in routine clinical settings, particularly in outpatient departments, remains limited.
More sophisticated tests, such as interferometry, meibography, dynamic interference lipid pattern tests, and meibomian gland expression, can identify problems with the lipid layer. These methods are typically reserved for severe DED because of their high cost, leaving a diagnostic gap for early or moderate stages of the disease.2 To bridge this gap, simpler screening methods for evaporative DED in outpatient departments can be employed. These methods combine signs and symptoms, occupational history, and basic screening tests,2,19 which do not require specialized equipment.
Blink test. Simple yet sensitive, the blink rate test is indicative of DED. According to TFOS DEWS II and Dry Eye Wheel criteria, a blink within 10 seconds while the patient focuses on a visual stimulus is suggestive of DED.20-22
Blink assessment. Parameters such as maximum blink interval, blink rate, and interblink interval can identify altered blinking patterns associated with evaporative DED. These can be measured with binocular wearable eye-tracking headsets,14 a front-facing iPad (Apple) camera,23 or mobile applications such as You Can Know Whether You Have Dry Eye in a Minute (Tsubo Labo)24 or DryEyeRhythm (Ohako and Medical Logue), specifically designed for DED screening.25,26
Detection of inflammatory markers. Tools such as InflammaDry (Quidel) measure levels of matrix metalloproteinase-9, which is elevated in DED, and can provide results within 10 minutes.27
Ocular protection index. This index evaluates the relationship between TBUT and blink interval. It is calculated by dividing TBUT by the mean interblink interval. During digital screen use, an increased blink interval coupled with a decreased TBUT results in an ocular protection index of less than 1, indicative of potential DED.3,11,28
4. PHARMACOLOGIC MANAGEMENT
Effective management of evaporative DED requires a comprehensive and proactive approach aimed at interrupting the cycle of DED by restoring and preserving the balance of the ocular surface system. This strategy involves a combination of preventive measures, behavioral changes, and both pharmacologic and nonpharmacologic interventions.2
Artificial tears. The cornerstone of DED management is artificial tears or tear substitutes.1 These are widely used by digital device users for symptomatic relief.6
Lipid-based eye drops mimic natural tears and are more effective than water-based options. The former can enhance tear film stability, decrease evaporation, and are generally better tolerated by patients with evaporative DED.2,29
Nanoemulsion-based lipid eye drops can improve lipid layer thickness, provide overall symptom relief,30,31 and reduce the signs of DED.32,33 These drops are thought to be superior to high-viscosity artificial tears.34
Nutritional supplements. Omega-3 fatty acid supplementation can address dry eye symptoms, reduce tear evaporation rates, and improve Nelson grades.6,35
5. NONPHARMACOLOGIC MANAGEMENT
Evaporative DED, particularly in the presence of MGD, can often be managed effectively with nonpharmacologic methods.
Blind viewing. Engaging in activities such as listening to music without reading (ie, blind viewing) can reduce eye strain.
Eye-warming devices. The delivery of consistent warmth can help treat MGD by ensuring the smooth flow of oils from the glands.
Lifestyle and workstation modifications. These include ergonomic exercises, adjusting workstation habits, and regulating the use of digital devices that can improve the signs and symptoms of DED.3
Thermal pulsation devices. This form of treatment can clear meibomian gland obstruction.2
Warm compresses and lid massages. These techniques can help alleviate meibomian gland obstruction by promoting oil secretion from the glands and stabilizing the tear film.36,37
1. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628.
2. Rolando M, Merayo-Lloves J. Management strategies for evaporative dry eye disease and future perspective. Curr Eye Res. 2022;47(6):813-823.
3. Kamøy B, Magno M, Nøland ST, et al. Video display terminal use and dry eye: preventive measures and future perspectives. Acta Ophthalmol. 2022;100(7):723-739.
4. Wolffsohn JS, Wang MTM, Vidal-Rohr M, et al. Demographic and lifestyle risk factors of dry eye disease subtypes: a cross-sectional study. Ocul Surf. 2021;21:58-63.
5. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-283.
6. Bhargava R, Kumar P, Phogat H, Kaur A, Kumar M. Oral omega-3 fatty acids treatment in computer vision syndrome related dry eye. Cont Lens Anterior Eye. 2015;38(3):206-210.
7. Courtin R, Pereira B, Naughton G, et al. Prevalence of dry eye disease in visual display terminal workers: a systematic review and meta-analysis. BMJ Open. 2016;6(1):e009675.
8. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802-812.
9. Wang MTM, Tien L, Han A, et al. Impact of blinking on ocular surface and tear film parameters. Ocul Surf. 2018;16(4):424-429.
10. Kaur K, Gurnani B, Nayak S, et al. Digital eye strain-a comprehensive review. Ophthalmol Ther. 2022;11(5):1655-1680.
11. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510.
12. Cardona G, Gómez M, Quevedo L, Gispets J. Effects of transient blur and VDT screen luminance changes on eyeblink rate. Cont Lens Anterior Eye. 2014;37(5):363-367.
13. McMonnies CW. Diagnosis and remediation of blink inefficiency. Cont Lens Anterior Eye. 2021;44(3).
14. Chidi-Egboka NC, Jalbert I, Chen J, Briggs NE, Golebiowski B. Blink rate measured in situ decreases while reading from printed text or digital devices, regardless of task duration, difficulty, or viewing distance. Invest Ophthalmol Vis Sci. 2023;64(2):14.
15. Wang MTM, Muntz A, Mamidi B, Wolffsohn JS, Craig JP. Modifiable lifestyle risk factors for dry eye disease. Cont Lens Anterior Eye. 2021;44(6):101409.
16. Yokoi N, Uchino M, Uchino Y, et al. Importance of tear film instability in dry eye disease in office workers using visual display terminals: The Osaka study. Am J Ophthalmol. 2015;159(4):748-754.
17. Shimazaki J, Seika D, Saga M, et al. A prospective, randomized trial of two mucin secretogogues for the treatment of dry eye syndrome in office workers. Sci Rep. 2017;7(1):15210.
18. Blehm C, Vishnu S, Khattak A, Mitra S, Yee RW. Computer vision syndrome: a review. Surv Ophthalmol. 2005;50(3):253-262.
19. Narang P, Donthineni PR, D’Souza S, Basu S. Evaporative dry eye disease due to meibomian gland dysfunction: preferred practice pattern guidelines for diagnosis and treatment. Indian J Ophthalmol. 2023;71(4):1348-1356.
20. Wolffsohn JS, Craig JP, Vidal-Rohr M, Huarte ST, Ah Kit L, Wang M. Blink test enhances ability to screen for dry eye disease. Cont Lens Anterior Eye. 2018;41(5):421-425.
21. Take the Optrex blink test now to see if you have signs of screen-dry eye. Pharmaceutical Journal. Accessed September 8, 2023. https://pharmaceutical-journal.com/wp-content/uploads/2021/02/1073671.pdf
22. Dry eye wheel. World Council of Optometry. Accessed December 1, 2023. https://dryeye.worldcouncilofoptometry.info/interactive-dry-eye-wheel/
23. Muntz A, Turnbull PR, Kim AD, et al. Extended screen time and dry eye in youth. Cont Lens Anterior Eye. 2022;45(5):101541.
24. Uchino M, Kawashima M, Uchino Y, et al. The evaluation of dry eye mobile apps for screening of dry eye disease and educational tear event in Japan. Ocul Surf. 2018;16(4):430-435.
25. Fujio K, Nagino K, Huang T, et al. Clinical utility of maximum blink interval measured by smartphone application DryEyeRhythm to support dry eye disease diagnosis. Sci Rep. 2023;13(1):1-10.
26. Okumura Y, Inomata T, Midorikawa-Inomata A, et al. DryEyeRhythm: a reliable and valid smartphone application for the diagnosis assistance of dry eye. Ocul Surf. 2022;25:19-25.
27. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-574.
28. Cardona G, Argilés M, Pérez-Cabré E. Loss of blink regularity and its impact on ocular surface exposure. Diagnostics (Basel). 2023;13(14):2362
29. Agarwal P, Craig JP, Rupenthal ID. Formulation considerations for the management of dry eye disease. Pharmaceutics. 2021;13(2):207.
30. Silverstein S, Yeu E, Tauber J, et al. Symptom relief following a single dose of propylene glycol-hydroxypropyl guar nanoemulsion in patients with dry eye disease: a phase IV, multicenter trial. Clin Ophthalmol. 2020(14):3167-3177.
31. Yeu E, Silverstein S, Guillon M, et al. Efficacy and safety of phospholipid nanoemulsion-based ocular lubricant for the management of various subtypes of dry eye disease: a phase IV, multicenter trial. Clin Ophthalmol. 2020;14:2561-2570.
32. Labetoulle M, Messmer EM, Pisella PJ, Ogundele A, Baudouin C. Safety and efficacy of a hydroxypropyl guar/polyethylene glycol/propylene glycol-based lubricant eye-drop in patients with dry eye. Br J Ophthalmol. 2017;101(4):487-492.
33. Ng A, Keech A, Jones L. Tear osmolarity changes after use of hydroxypropyl-guar-based lubricating eye drops. Clin Ophthalmol. 2018;12:695-700.
34. Weisenberger K, Fogt N, Swingle Fogt J. Comparison of nanoemulsion and non-emollient artificial tears on tear lipid layer thickness and symptoms. J Optom. 2020;14(1):20-27.
35. Pellegrini M, Senni C, Bernabei F, et al. The role of nutrition and nutritional supplements in ocular surface diseases. Nutrients. 2020;12(4):952.
36. Goel P, Zadeh O, Benjamin Kambiz Ghiam HDH. Treating evaporative dry eye associated with meibomian gland dysfunction. EyeNet Magazine. 2019. Accessed January 9, 2024. https://www.aao.org/eyenet/article/treating-evaporative-dry-eye
37. Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050-2064.




