The decision to add MIGS to my surgical practice was an easy one: a number of my patients were coming to me for cataract services who also had mild to moderate glaucoma. My thinking was, if I was already going to do surgery on the lens, I had an opportunity to address both problems during a single operation.
However, I would not have added any MIGS device or procedure to my armamentarium had the surgical steps been difficult. More importantly, I had to know that whatever intervention I was going to offer would truly have a benefit for my patients. Each of these concerns—whether it would interrupt my surgical flow and whether it worked—are what led me to learn more about canaloplasty, and more specifically about the iTrack Advance (Figure 1) from Nova Eye Medical.

Figure 1. The iTrack Advance is designed to be an all-in-one handpiece, adding greater ergonomics and better intraprocedural control.
The Evolution of Canaloplasty
I first discovered iTrack when it was designed as an ab externo surgery. I immediately appreciated that the procedure was mechanically breaking herniations in the Schlemm canal (SC) while also introducing titratable volumes of OVD to clear the SC, trabecular meshwork (TM), and collector channels. Frankly, I also thought the technology was pretty ingenious, especially the illuminated microcatheter that provided real-time feedback to the surgeon on its location within the canal.
Fast forward to today, and iTrack has evolved significantly. The same fundamental steps are performed, but from an ab interno approach, making is suitable for use during cataract surgery; yet, because it is a minimally invasive procedure, it is also a viable option for a standalone surgery to address uncontrolled glaucoma.

More recently, the company has released a second generation of its core platform, the iTrack Advance, which introduces features intended to make the device even more user friendly and easier to integrate into the surgical workflow. It has equivalent safety and efficacy as the original iTrack, but the handpiece has been designed to be an all-in-one cannula and injector, with a smooth gliding mechanism for precise advancement and retraction of the microcatheter. Eliminating the need for instrument passes compared to the first-generation device makes the iTrack Advance easier to use and teach to new surgeons.

Key Features
There are at least three features of the iTrack Advance that have stood out to me as I have performed cases and worked with training surgeons. The first is that iTrack Advance is the only canaloplasty or viscodilation technology on the market that allows up to 360° of catheterization in a single pass. A complete catheterization can be achieved with other devices; however, the surgeon has to advance the microcatheter 180° in one direction, reverse, and then advance in the opposite direction. Related to this is the second feature: The iTrack Advance also offers a rotating nozzle, making it suitable for use by right- and left-handed surgeons alike.
Third, viscoelastic delivery is controlled by a technician or surgical staff member rather than being a continuous flow. In our OR, I simply count aloud as I am retracting, signaling to my scrub nurse to eject a controlled amount of viscoelastic. It’s a simple system that allows me to titrate exactly how much viscoelastic is delivered to each quadrant of the eye. And, because someone else is pushing the button to eject OVD, my hands and attention are free to concentrate on the case.

Trimodal MoA
The real advantages of iTrack Advance are less so in how it does what it does, and more so in what it accomplishes with a single procedure.
It is understood that a significant portion of resistance in primary open-angle glaucoma (POAG) is in the TM,1 so in that respect it makes sense to implant devices or ablate the meshwork tissue to affect greater flow. However, there is also growing evidence that the outflow pathway is complex, and that herniations or blockages in other parts of the system—such as the SC or the collector channels—are relevant for driving POAG-associated pathology. There is also evidence that the TM participates in regulating pressure spikes, and so damaging or removing the meshwork would impede an important physiologic function.
The steps of the procedure are straightforward; I typically perform iTrack Advance before lens removal when I know I will have good visualization and the anterior chamber is still firm. Over the entire procedure, the iTrack Advance procedure targets three distinct parts of the aqueous drainage pathway, thereby offering a trimodal mechanism that addresses the underlying anatomy.
In the first step, the microcatheter is introduced to the SC through a sideport incision (as small as 1.0 mm for standalone procedures) and advanced for up to 360°; the microcatheter mechanically breaking herniations in the SC is the first mechanism. The lighted tip of the microcatheter gives instant feedback that the microcatheter is in the SC, and should there be any impassable obstructions, it is easy to reverse, pivot the head on the probe, and advance in the opposite direction, again with confirmation that the tip is being directed around the SC (Figure 2).
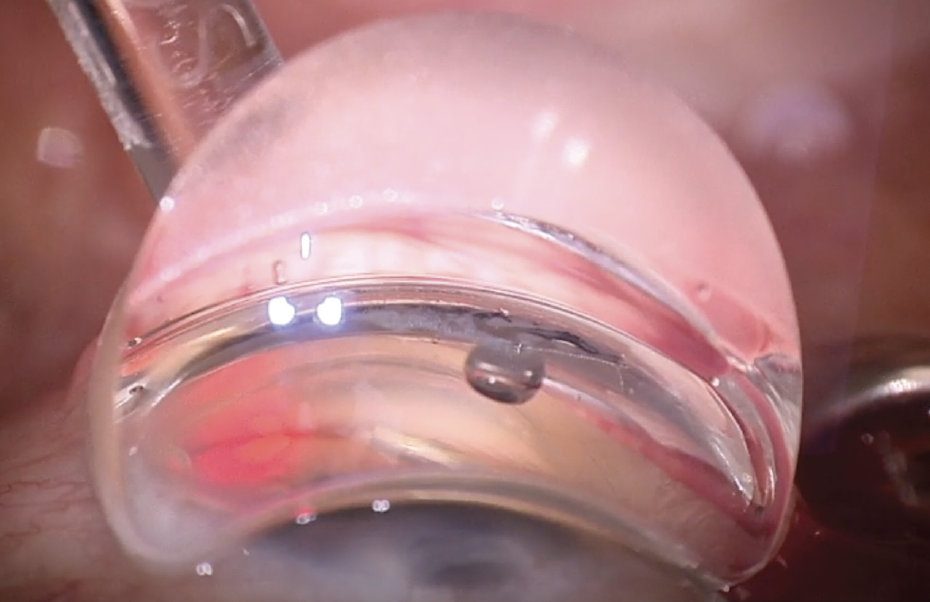
Figure 2. The blinking red beacon at the tip of the iTrack Advance catheter gives the surgeon
real-time feedback on its location.
Photo Courtesy of I. Paul Singh, MD
In the next part of the procedure, the microcatheter is retracted as pressurized viscodilation is performed. This step, which introduces the second mechanism, addresses the fact that the inner lumen of the SC is often smaller in POAG eyes, accounting for up to 50% of decreased outflow facility.2,3 In addition to breaking adhesions in the SC, viscodilation helps dilate the canal.4-6 Notably, there is often instant confirmation of effect, with blanching of the vessels apparent intraoperatively (Figure 3). As well, pressurized viscodilation also has the function of stretching the TM, creating tiny microperforations in the meshwork to improve outflow facility. As a third mechanism, the pressurized dilation also flushes the collector channels, which are blocked by herniation in up to 90% of eyes with POAG.7,8

Figure 3. Intraoperative photos of a patient undergoing an iTrack Advance procedure, before (A) and after (B) introduction of viscoelastic. Note the blanching of vessels around the limbus.
Photo Courtesy of I. Paul Singh, MD

Restoring Physiologic Outflow
Taken together, the iTrack Advance procedure is a 360° treatment to restore natural aqueous flow in the conventional drainage pathway by addressing the anatomy in at least three areas. We currently have no way of measuring where obstructions are occurring, so it makes intuitive sense to offer a comprehensive approach to restoring physiologic drainage. If we are able to do so while maintaining future treatment options and without compromising important functions of the TM in regulating IOP, all the better.
The fact that iTrack Advance is a 360° treatment with trimodal effect (and with titratable viscoelastic delivery) stands in contrast to stent procedures, which are focal treatments by design, directing flow at one distinct point. However, if a stent is placed near a clogged collector channel, there is potential for no benefit. With the iTrack Advance procedure, because we are targeting and treating all possible points of outflow resistance, we are comprehensively treating the disease and therefore increasing our odds of a better clinical outcome. The titratable nature of the procedure also makes it versatile, suitable for a wide range of patients.


In my view, any patient with open-angle glaucoma could benefit from an iTrack Advance procedure. I routinely offer the option to my patients with cataract and co-existing glaucoma, which is historically about 20% of the cataract population. As an added extra, knowing that I have done something to restore physiologic outflow gives me confidence in the postoperative cataract period that I am guarding against pressure spikes.
1. Manik Goel, Renata G Picciani, Richard K Lee, and Sanjoy K Bhattacharya. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010; 4:52-59.
2. Johnstone MA, Grant WG. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75:365-383
3. Allingham RR, de Kater AW, Ethier CR. Schlemm’s canal and primary open angle glaucoma: correlation between Schlemm’s canal dimensions and outflow facility. Exp Eye Res. 1996;62(1):101-109.
4. Stegmann R, Pienaar A, Miller D. Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refract Surg. 1999;25(3):316-322.
5. Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Clinical evaluation of the aqueous outflow system in primary open-angle glaucoma for canaloplasty. Invest Ophthalmol Vis Sci. 2010;51(3):1498-1504.
6. Smit BA, Johnstone MA. Effects of viscoelastic injection into Schlemm’s canal in primate and human eyes: potential relevance to viscocanalostomy. Ophthalmology. 2002;109(4):786-792.
7. Battista SA, Lu Z, Hofmann S, et al. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49(12):5346-5352.
8. Gong H and Francis A: Schlemm’s Canal and Collector Channels as Therapeutic Targets. In Innovations in Glaucoma Surgery, Samples JR and Ahmed I eds. Chapter 1, page 3 – 25, Springer New York, 2014.
The iTrack™ Advance has a CE Mark (Conformité Européenne) and US Food and Drug Administration (FDA) 510(k) #K221872 for the treatment of open-angle glaucoma.
INDICATIONS: The iTrack™ Advance is indicated for fluid infusion or aspiration during surgery. The iTrack™ Advance is indicated for catheterization and viscodilation of Schlemm’s canal to reduce intraocular pressure in adult patients with open angle glaucoma.
CONTRAINDICATIONS: The iTrack™ Advance is not intended to be used for catheterization and viscodilation of Schlemm’s canal to reduce intraocular pressure in eyes of patients with the following conditions: Neovascular glaucoma; Angle-closure glaucoma; Previous surgery with resultant scarring of Schlemm’s canal.
ADVERSE EVENTS: Possible adverse events with the use of the iTrack™ Advance include, but are not limited to: hyphema, elevated IOP, Descemet’s membrane detachment, shallow or at anterior chamber, hypotony, trabecular meshwork rupture, choroidal effusion, Peripheral Anterior Synechiae (PAS) and iris prolapse.
PRECAUTIONS: The iTrack™ Advance should be used only by physicians trained in ophthalmic surgery. Knowledge of surgical techniques, proper use of the surgical instruments, and post-operative patient management are considerations essential to a successful outcome.
For full safety information, visit: https://itrack-advance.com/us




