AcrySof IQ PanOptix
This lens boasts good visual and patient satisfaction results.
Parag A. Majmudar, MD

About 18 months ago, I was honored to be invited to Canada to speak at an ophthalmology conference there. I remember discussing with other attendees the status of premium lenses in the United States and current practices in Canada. There was an overwhelming response from my international colleagues that the AcrySof IQ PanOptix Trifocal IOL (Alcon; Figure 1) was quickly becoming the go-to lens for presbyopia correction for those Canadian surgeons and their patients.

Figure 1. The PanOptix IOL, the first trifocal IOL available in the United States.
Courtesy of Alcon
This was both a little surprising, because international surgeons have access to more platforms than we are used to in the United States, and exciting, as I had heard rumblings that the PanOptix IOL had completed its US clinical trials and was awaiting imminent FDA approval. Less than 1 year later, American surgeons had access to the lens, and I had the opportunity to implant the PanOptix, the first trifocal IOL available in this country, in a number of patients beginning in September 2019.
The PanOptix is based on the familiar Alcon AcrySof hydrophobic acrylic IOL platform, which has been implanted in more than 120 million eyes worldwide. The lens uses Alcon’s Enlighten Optical Technology, which, according to the company, provides exceptionally high light utilization (88%) with less pupil dependence than other multifocal IOL technologies. This enables the lens to improve intermediate visual acuity without degrading distance and near acuity. Because it is available in toric configurations, it is indicated for the correction of a wide range of refractive errors.
The lens, as I mentioned, has been well received internationally. In the FDA study, the results for intermediate and near visual acuities compared to control eyes were impressive. The lens also earned a high rate of patient satisfaction, with more than 99% of patients indicating that they would choose the same lens again.1
EARLY RESULTS
My early results with PanOptix have been excellent, and the vast majority of patients have been satisfied with their results. I have not yet had to explant a PanOptix lens.
Key factors that lead to a successful result include ensuring refractive accuracy, correcting preexisting astigmatism, and performing proper patient selection from a physical as well as psychometric perspective. I have found that patients are less happy when they get a slightly hyperopic postoperative result (0.25 D), so my target tends to be plano to slightly myopic (-0.25 D). If these conditions are met, most patients achieve very good uncorrected distance visual acuity (20/20 to 20/25) as well as J1 or J2 near and intermediate visual acuity.
NO PERFECT LENS
As with all currently available multifocal lenses, I explain to my patients that excellent results can be achieved with the PanOptix but also stress that no lens is, at this moment, perfect. I advise patients that visual disturbances such as glare, halo, or starbursts may be present. As we have seen with previous generations of multifocal IOLs, there may be a period of neural adaptation. Most patients are willing to put up with these tradeoffs to achieve improved spectacle independence.
Many US surgeons are excited about the PanOptix Trifocal IOL. We have been pleased with early visual acuity and patient satisfaction results, and we remain cautiously optimistic that results will be maintained for many decades.
1. AcrySof IQ PanOptix Directions For Use.
2. Alcon introduces AcrySof IQ PanOptix trifocal IOL in the US, the first and only FDA-approved trifocal lens. Eyewire. https://eyewire.news/articles/alcon-introduces-acrysof-iq-panoptix-trifocal-iol-in-the-us-the-first-and-only-fda-approved-trifocal-lens/. Accessed March 27, 2020.
enVista Toric
Wide range of cylinder powers expands options for astigmatism correction.
Audrey R. Talley Rostov, MD

The goal of every cataract surgeon is to provide the best possible visual outcome for all patients. For me, that includes addressing astigmatism at the time of surgery whenever possible. Astigmatism as little as 0.75 D has the potential to affect functional and low-contrast acuity,1 and nearly 75% of cataract patients have astigmatism above this threshold.2
The enhanced enVista MX60ET hydrophobic acrylic toric IOL (Bausch + Lomb; Figure 2), available in cylinder powers as low as 1.25 D, allows me to offer an enhanced range of astigmatic correction as low as about 0.77 D at the corneal plane. This lens and its toric sister, the enVista MX60T (Bausch + Lomb), are currently the only IOLs available in the United States that can correct less than 1.00 D of corneal astigmatism.

Figure 2. The enhanced enVista MX60ET hydrophobic acrylic toric.
Courtesy of Bausch + Lomb
All enVista IOLs are aspheric, but they do not add spherical aberration to compensate for the physiologic corneal spherical aberration. It is well known that spherical aberration in the cornea, which is left intact by enVista IOLs, can provide some range of vision.
GOOD IN both ROUTINE and COMPLEX CASES
Because the optical power of the MX60ET is equal from center to edge, it is not sensitive to the effects of decentration or tilt, and it provides a desirable compromise between range of vision and image quality. I believe this makes enVista a user-friendly option for both routine and complex patients.
As a cornea specialist, I see a significant number of complex patients who have corneal abnormalities, previous radial keratotomy or LASIK surgery, or even corneal transplants who want to optimize their vision and improve both their refractive and functional results. For these patients in particular, the enVista’s aspheric optic provides an option that doesn’t add aberration to an already aberrated cornea. This contributes to predictability in achieving desired refractive outcomes.
LENS DESIGN
Like all monofocal and toric enVista options, the MX60ET features an aspheric optic design, step-vaulted haptics, and a continuous 360° square edge. The broad capsular contact of the enVista haptics aids in securing the lens position and ensures excellent rotational stability.
The enVista IOLs also remain the only one-piece hydrophobic acrylic IOLs available in the United States that are glistening-free. The optic is so clear that, when I started using these lenses, my retina specialist colleagues would often ask me what I had implanted in a referred patient because their view was so clear when performing procedures such as epiretinal membrane surgery.
Finally, the MX60ET incorporates improved material properties designed to enhance recovery of the optic, which may help contribute to greater surgical efficiency.
CONCLUSION
Overall, the enVista MX60ET gives me the ability to correct astigmatism for more patients—including those with both complex and routine presentations—and adds a level of confidence that I’ll be able to get them to their best possible vision.
1. Miller AD, Kris MJ, Griffiths AC. Effect of small focal errors on vision. Optom Vis Sci. 1997;74(7):521-526.
2. Prevalence of corneal astigmatism prior to cataract surgery. East Valley Ophthalmology (Warren Hill, MD, database). http://www.doctor-hill.com/physicians/docs/Astigmatism.pdf. Accessed March 17, 2020.
Light Adjustable Lens
What I’ve learned thus far: Promising early results have led to routine excellence in clinical experience.
Kevin L. Waltz, OD, MD

What a difference knowledge and technology make. I began my surgical career in the 1980s, performing large-incision extracapsular cataract extraction. When I first saw Howard V. Gimbel, MD, performing a capsulorhexis and phacoemulsification, I was amazed. He was a magician in surgery. He could do things few others could do.
Of course, there have been many advances in cataract surgery over the past 30 years, and we are all surgical magicians now by the standards of cataract surgery in the 1980s. It is a wonderful time to be a cataract surgeon.
Want to further improve your magic? We now have the Light Adjustable Lens (RxSight; Figure 3) to offer our patients. It is as close to magic as I have seen in my clinical practice. It looks like a three-piece silicone IOL from the 1990s on my surgical tray and in patients’ eyes, but these patients see so well with it that you may not believe my story.

Figure 3. The Light Adjustable Lens has a square posterior edge and minimal negative and positive dysphotopsias.
Figures 3 through 5 courtesy of RxSight
DISRUPTIVE TECHNOLOGY
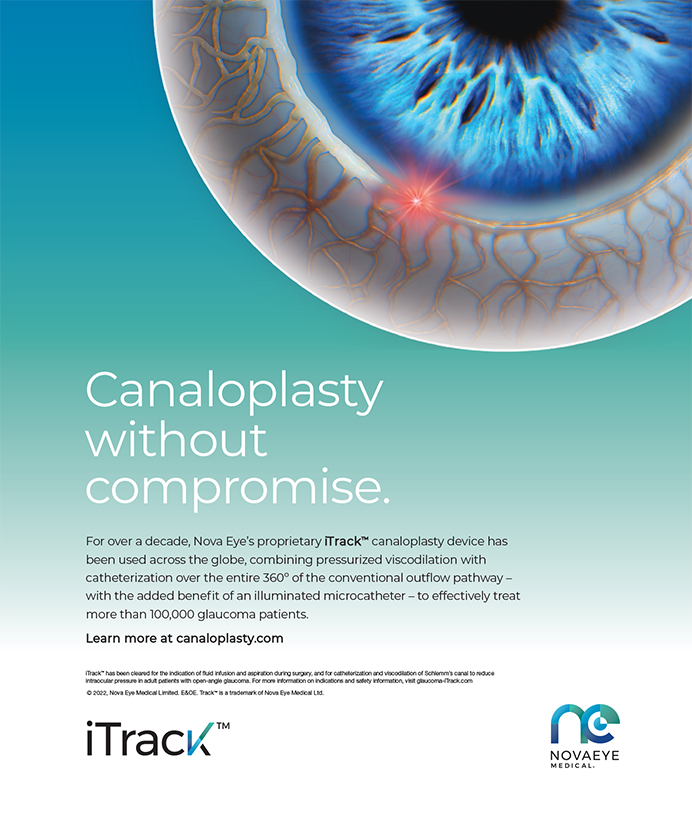
A clever surgeon described the Light Adjustable Lens as disruptive, both in the outcomes it provides to the patient and in the processes used by doctors and staff to achieve that outcome (Figure 4). I agree.
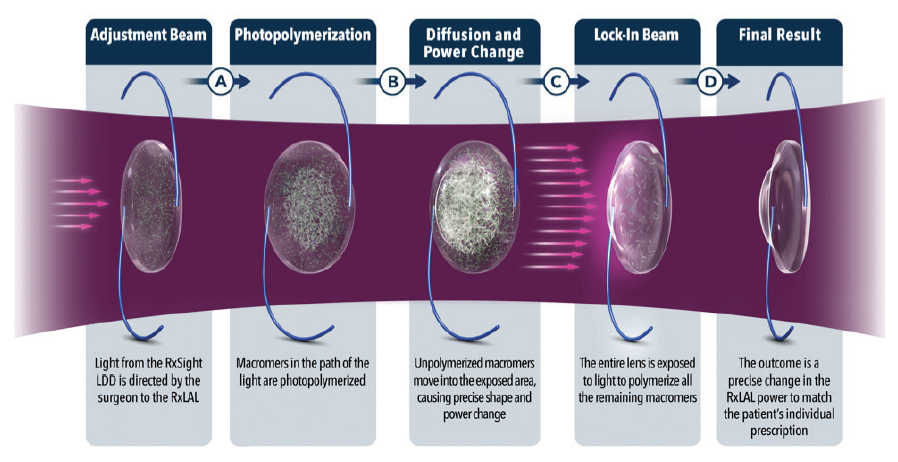
Figure 4. Schematic of the photochemical treatment process.
Outcomes. I participated in the US clinical trial that led to FDA approval of the Light Adjustable Lens in 2017.1 The company used the toric IOL pathway to approval, starting in approximately 2012, and I implanted a number of Light Adjustable Lens IOLs during that time. The FDA trial demonstrated excellent results with very good reduction in treated astigmatism, no IOL rotational issues, and excellent UCVA after adjustment, with a sharp reduction in residual spherical error after adjustment.
The most surprising finding from the FDA trial was the patients who achieved good uncorrected near visual acuity (UNVA) when their uncorrected distance visual acuity (UDVA) was excellent. This did not happen in every patient, but it was not uncommon when we achieved both excellent UDVA of 20/15 to 20/20 and excellent UNVA of J1 to J5 in the same eye, with a theoretically monofocal IOL.
Processes. Although the Light Adjustable Lens was cleared for US marketing by the FDA in November 2018, it was not released by RxSight for commercial use until the summer of 2019. I started using the Light Adjustable Lens commercially in September of that year. I started slowly for a variety of reasons, even though I was very familiar with the technology from the trial. My reasons for going slowly included:
- Accounting for modifications in the process and outcomes when we transitioned from a trial to commercial use;
- I was in a new practice setting;
- The lens is relatively expensive compared to other toric and presbyopia-correcting IOLs; and
- It took a lot of thinking and clinical work for each IOL, and I had to improve my workflow to adapt.
I implanted several Light Adjustable Lenses during that fall of 2019. In the first 10 patients, not a single one achieved less than 20/20 UDVA in both eyes. Most patients achieved 20/16 or better and also had good UNVA of J1+ to J5. The results were similar to the wow factor we experienced with LASIK in the 1990s.
On balance, achieving these results with the Light Adjustable Lens required time, effort, and thought—and a precise refraction prior to every treatment. I priced the services in such a way that I was happy to provide the work needed.
A typical patient in my practice required two to three adjustments and then a final lock-in treatment to stop the lens from changing any further. Each treatment required a focused refractive surgery discussion, a very accurate manifest refraction, strong dilation, and treatment with the Light Delivery Device (LDD, RxSight; Figure 5). From beginning to end, it was about 45 minutes of contact time for each patient for each treatment. Not all of that time was spent with me, but I needed a great staff to help me provide the services without drowning.
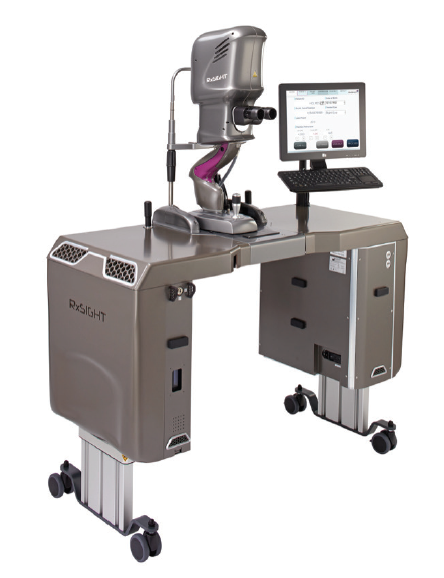
Figure 5. The Light Delivery Device is about the size of an Nd:YAG laser.
Careful planning of my week was necessary to provide all the treatments required. It was helpful to my efficiency to treat both eyes together; it is impractical to treat one eye with the Light Adjustable Lens followed by the other eye on a different schedule.
WHAT TO EXPECT
I tell all my patients preoperatively that they will achieve great UDVA postoperatively. I tell them that the typical result is a UDVA of 20/16. I show them the 20/16 line on the wall chart and tell them that is our goal. This is the same thing I tell refractive surgery patients: “You will have little to no refractive error, and you will see great far away. In fact, being a previous refractive surgery patient is one of the best indications for having a Light Adjustable Lens implanted.”
So far, I have not missed my refractive target with the Light Adjustable Lens in the past 6 months, and several of my patients who had previously had laser vision correction who now have the Light Adjustable Lens have achieved 20/10 and J1+ results uncorrected. I don’t promise patients that, but I am not surprised when it happens.
A GREAT CASE
The following is a great patient example. Jeffrey J. Machat, MD, FRCSC, DABO, taught many of us how to offer laser vision correction during the past 25 years. He performed my wife’s LASIK in 1996. I was honored when he flew to Indianapolis to have me implant a Light Adjustable Lens in his right eye and returned for all of the LDD treatments, which were performed at Whitson Vision.
During the preoperative discussion, I told Dr. Machat that he would have at least 20/16 UDVA when we were done. He reminded me that he had corneal coma and told me not to overpromise the results—as he had taught me and others long ago. I explained to him that I was not overpromising. With the Light Adjustable Lens, I said, I knew I could provide that level of vision.
Today, Dr. Machat has 20/10 UDVA and is thrilled. Such is the power of the Light Adjustable Lens, that you can make a commitment to a colleague, family member, or friend and know that it will happen. The Light Adjustable Lens is as close to magic as I have seen in my clinical practice.
1. FDA approves first implanted lens that can be adjusted after cataract surgery to improve vision without eyeglasses in some patients [press release]. US Food and Drug Administration. November 22, 2017. https://www.fda.gov/news-events/press-announcements/fda-approves-first-implanted-lens-can-be-adjusted-after-cataract-surgery-improve-vision-without. Accessed March 19, 2020.
Tecnis Toric II
First global commercial experience.
George O. Waring IV, MD, FACS

Astigmatism affects nearly half of patients undergoing cataract surgery and increases in prevalence with age (Figure 6).1 However, it often goes uncorrected at surgery, leaving patients dependent on glasses afterward.
The best way to correct astigmatism so that patients can achieve good distance UCVA after cataract surgery is the use of toric IOLs, but financial aspects and physicians’ concerns about achieving proper alignment and maintaining rotational stability have been barriers to their more widespread implementation. The same can be said for the larger category of lenses that we typically refer to as premium IOLs.
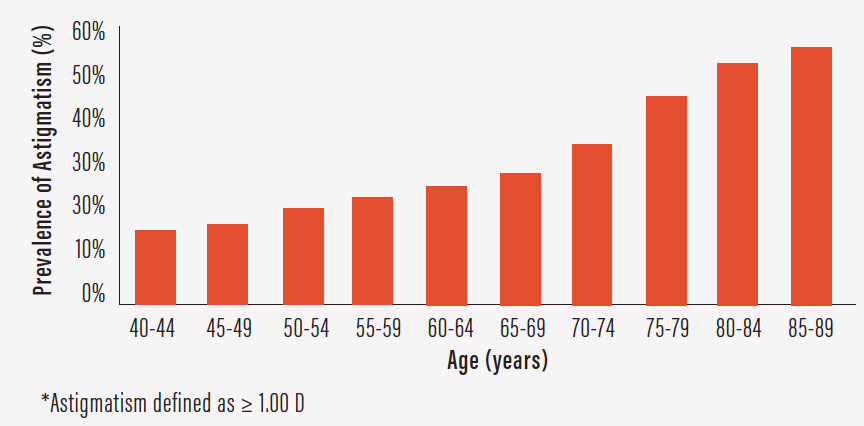
Figure 6. Prevalence of astigmatism increases with age.
After working with Johnson & Johnson Vision for the past several years on ongoing refinements in its toric IOL platform, I recently had the opportunity to implant the first commercial Tecnis Toric II (ZCU) lenses in the world (Figure 7). I have now implanted many of these lenses and have spoken to other surgeons around the country about their initial experiences, which I will relate here.
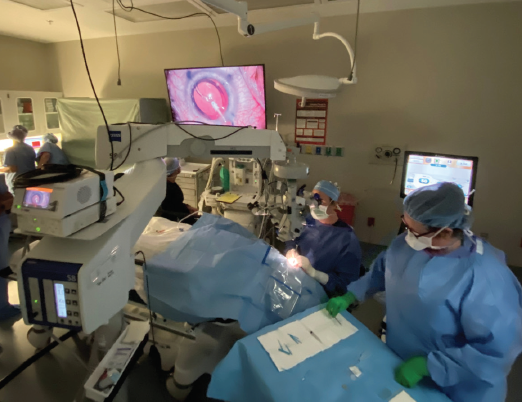
Figure 7. Dr. Waring (seated) performed the world’s first commercial Tecnis Toric II implantation.
Courtesy of George O. Waring IV, MD, FACS
DISTINGUISHING FEATURES
As with any toric IOL, successful implantation of the Tecnis Toric II requires meticulous biometry and preoperative measurement of astigmatism axis and power. For best results, the surgeon should take both posterior corneal astigmatism and surgically induced astigmatism into account.
Intraoperatively, surgeons will find that the Tecnis Toric II is similar in many ways to its predecessor, the Tecnis Toric (ZCT). No changes have been made to the optical design of the lens, so it features the same optics as other Tecnis IOLs. This lens platform delivers high-quality vision because of its minimal chromatic aberration, correction of spherical aberration to nearly zero, index of refraction that reduces surface reflectance, low propensity for capsular phimosis, and lack of association with glistenings.
What makes the new version of this toric IOL unique is its haptic design, which features haptics that are slightly more squared and frosted than the ZCT’s haptics (Figure 8). The subtle texture of the haptics adds stability within the capsular bag, with the goal of further minimizing the already low rate of rotation.
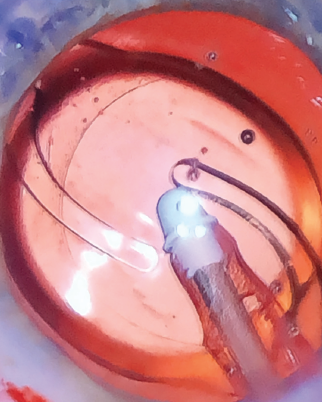
Figure 8. The ZCU’s haptics are slightly more squared and frosted than those of the predecessor lens.
In clinical trials that led to FDA marketing approval in December 2019, the Tecnis Toric II demonstrated excellent rotational stability. The company is undertaking two postmarket clinical trials that will include up to 1,000 patients.
INTRAOPERATIVE RECOMMENDATIONS
Surgeons who are accustomed to the Tecnis Toric lens should familiarize themselves with the differences and make some minor adjustments in technique when using the Tecnis Toric II IOL. Unlike the Tecnis Toric lens, which could be freely rotated either clockwise or counterclockwise to align the lens with active irrigation, surgeons may notice the intentional difference in the stability of the Tecnis Toric II once the haptics have deployed. Therefore, the design features that contribute to greater stability also require a different approach to manipulating the lens.
My three recommendations for lens manipulation are:
No. 1: Completely fill the capsule with a cohesive OVD before injecting the IOL;
No. 2: Align the lens on the correct axis or just shy of the correct axis immediately, before the haptics are fully deployed; and
No. 3: Take care when rotating the Tecnis Toric II after the haptics are deployed by using more conservative rotation and paying attention to any potential resistance.
My preference is to align perfectly at the intended axis of astigmatism correction. If any lens movement occurs during removal of the OVD, the conservative approach would be to consider a full rotation clockwise. Some surgeons may choose to under-align by a few degrees to account for lens jump, but I have not found this to be necessary.
Once the lens is stable and in the correct position, the OVD can be removed from behind the lens. As the last of the OVD is removed, I apply slight posterior pressure on the implant to hold it in position. If additional rotation is required, the capsule should be generously refilled with a cohesive OVD, and then the lens can be slowly dialed into the correct position in a clockwise direction. Conservative rotation under a fluid fill can also be considered.
The surgeon should also have a low threshold for use of a haptic tuck technique, in which the haptic is engaged with the second instrument and pulled centrally to disengage it from the fornix of the capsule. In this maneuver, I engage the haptic-optic junction and rotate the lens with a conservative, gentle, lateral rotation with the I/A tip or second instrument before removing the cohesive OVD. This frees the stability features of the haptics, making it easier to rotate the lens into position.
I have observed exceptional rotational stability with the Tecnis Toric II IOL. Once placed in the correct axis, the lens tends to remain quite stable.
CONCLUSION
We still have much to learn about factors that influence rotational instability, in particular the relationships between anatomy and lens position. Our group has studied and published work on capsular and lenticular morphology, demonstrating significant anatomic variability.2 Given this variability, intraoperative image guidance may have a role to play in toric IOL selection and positioning in the future.
The Tecnis Toric II is a fine example of the iterative improvements in IOL design and performance that continue to benefit surgeons and, most important, our patients.
1. Williams KM, Verhoeven VHM, Cumberland P, et al. Prevalence of refractive error in Europe: The European Eye Epidemiology (E(3)) Consortium. Eur J Epidemiol. 2015;30(4):305-315.
2. Haddad JS, Rocha KM, Yeh K, Waring GO 4th. Lens anatomy parameters with intraoperative spectral-domain optical coherence tomography in cataractous eyes. Clin Ophthalmol. 2019;13:253-260.