AcrySof IQ Vivity
Natural vision at a range of distances provided by a novel optical technology.
Edoardo Ligabue, MD

Recently, the increasing number of advanced-technology IOLs available for the correction of presbyopia and astigmatism has changed my approach to patient management. In my practice, I need to understand a patient’s visual needs in order to provide him or her with the best surgical treatment.
Every decision requires consideration of the patient’s ocular health status, including the presence of regular astigmatism, the absence of pathologic conditions such as maculopathy or glaucoma, and a good tear film. For a long time, there wasn’t much problem choosing the right IOL because most IOLs were monofocal. They were not often associated with issues such as glare, low contrast, and other visual disturbances.
Now, the AcrySof IQ Vivity IOL (Alcon; Figure 1) is providing vision like that of a monofocal IOL, with clear visual acuity at a range of distances, without the drawbacks of existing multifocal IOL technologies. This innovation provides a newfound stability in IOL selection and new possibilities in visual correction.
Figure 1. The AcrySof IQ Vivity IOL.
Courtesy of Edoardo Ligabue, MD
NATURAL VISION
From my first experience with the Vivity, its distinguishing features have been its ease of use and a visual disturbance profile like that of a monofocal IOL. In my experience, patients with the Vivity have natural vision with a continuous extended focal range from distance to intermediate (66 cm) to functional near; in bright light, they can easily read up to J3, and they report high satisfaction.
The Vivity is pupil-independent and is not affected by angle kappa. Contrast sensitivity, distance visual acuity, glare, and night vision quality are comparable to the characteristics of a monofocal IOL.
The Vivity does not use diffractive or refractive technology to provide presbyopia correction. Instead, the lens uses Alcon’s novel X-Wave technology, which is completely different from the technologies used by all other IOLs in the market. The X-Wave technology creates an extended focal range by stretching and shifting the wavefront, as opposed to splitting the wavefront into multiple focal points as diffractive multifocal lenses do.
By using all available light, this IOL maintains the same vision quality as a monofocal IOL: excellent distance vision, intermediate sharp vision for active lifestyles, and good quality vision for close-up daily activities (Figure 2).1
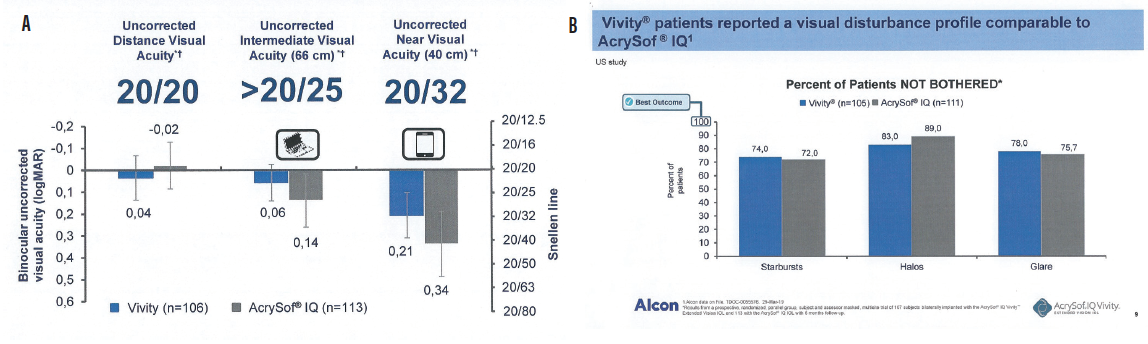
Figure 2. Uncorrected vision (A) and visual disturbance (B) results from a prospective, randomized, parallel-group, patient and assessor masked, multisite trial of 107 patients bilaterally implanted with the AcrySof IQ Vivity IOL and 113 with the AcrySof IQ IOL with 6-month follow-up. AcrySof IQ Vivity Alcon data on file (TDOC-0055576, March 29, 2019).
Courtesy of Alcon
CLINICAL EXPERIENCE
The results shown in Figure 2, from the first clinical studies of the AcrySof IQ Vivity, are in line with the results I have seen with my patients in my first 4 months implanting the Vivity.
When I propose a diffractive IOL to my patients, I always ask them if they can accept loss of a little bit of distance visual quality to gain independence from spectacles. But when I discuss the Vivity IOL, offering excellent distance visual quality with occasional spectacle use to read small text, the choice is unanimous: Quality of vision is patients’ chief preference.
The AcrySof IQ Vivity allows me to have no worries about refractive outcomes. In my experience, it is forgiving regarding biometric accuracy, it is not sensitive to tear film variations, and it does not impair visual acuity in patients with maculopathies or glaucoma. The lens provides stability in the capsular bag and is tolerant to slight decentration. The Vivity is also available in a toric IOL model, allowing patients to achieve astigmatism correction at the time of cataract surgery.
CONCLUSION
The Vivity is commercially available in selected European markets at present. Availability in additional countries will follow throughout 2020, according to Alcon. Based on my experience to date, the AcrySof IQ Vivity IOL will soon become the first IOL choice for most of my patients. It has changed the game by improving patients’ visual performance, making my job easier and more relaxing.
1. Data on file. Alcon.
FineVision Triumf
Improved vision and contrast sensitivity are associated with the lens’ chromatic aberration-free optical design.
Robert Edward Ang, MD

The FineVision Triumf (PhysIOL; Figure 3), recently introduced internationally, is a trifocal IOL with extended depth of focus (EDOF) optical technology. I was one of the first two surgeons worldwide to implant the Triumf IOL, and I am now participating in a prospective multicenter study of the lens.

Figure 3. The FineVision Triumf IOL.
The Triumf is made of PhysIOL’s proprietary glistening-free hydrophobic acrylic lens material. The overall diameter and the optic diameter of the Triumf are 11.4 and 6 mm, respectively, and the lens is UV- and blue light–filtering. This article reviews some of the early results and 6-month follow-up data on 78 eyes of 40 patients implanted with the lens.
The mean age of patients was 65.6 years. Of the 40 patients, 18 were Asian and 22 were white. This is important because Asian eyes tend to be more myopic, and this study is providing valuable information on how the IOL behaves in both populations. We used a target refraction of plano to -0.25 D for two reasons. First, we wanted the sphere to be consistently on target to calculate a universal A-constant, and second, a slight myopic spherical equivalent helps patients to achieve better near vision.
RESULTS
The mean monocular uncorrected distance visual acuity (UDVA) in these 78 eyes was 20/22, with 75% of patients achieving 20/20 or better and 79% achieving 20/25 or better. Mean monocular best corrected distance visual acuity (BDVA) was 20/20, with 11% of eyes achieving 20/16 or better and 96% achieving 20/20 or better.
It is unusual to obtain results this good on a multifocal IOL platform. We attribute these results to the chromatic aberration characteristics of the Triumf, which I will discuss further later in this article.
Mean monocular uncorrected intermediate visual acuity (UIVA) at 6 months postoperative was 20/23, with 46% of patients achieving 20/20 or better and 89% achieving 20/25 or better UIVA. When patients were corrected for distance, the mean UIVA was approximately 20/22, with 64% of patients achieving 20/20 or better and 89% achieving 20/25 or better.
The mean monocular uncorrected near visual acuity (UNVA) with the Triumf was 20/25. Further, 46% of patients achieved 20/20 or better and 68% achieved 20/25 or better. This is an excellent result, showing that near vision is not sacrificed with the Triumf, even as distance and intermediate acuities remain strong. When the influence of the slight myopia was corrected, about 75% of patients still achieved 20/25 distance-corrected near visual acuity (DCNVA).
GOOD VISION AT ALL DISTANCES
From these results, we determined that mean visual acuity through all distances can be expected to be about 20/25.
The defocus curves, based on visual acuity outcomes from distance, intermediate, and near visual acuity tests, showed that the lens provides patients with a broad range of vision (monocular: -3.20 to +1.30 D [31 cm to infinity]; binocular: -3.80 to +1.50 D [26 cm to infinity]).
Patients enrolled in the study to date have been highly satisfied. All patients reported spectacle independence at distance and intermediate, and 95.7% reported spectacle independence at near. Only 4.3% of patients reported needing glasses for near vision tasks some of the time, and none reported needing them all or most of the time.
CONCLUSION
We attribute the improved vision and contrast sensitivity with the Triumf to its chromatic aberration-free optical design, which corrects longitudinal chromatic aberration. The major difference in the design of the Triumf, in comparison with the FineVision trifocal IOL, is the height of the steps in its dual bifocal elements. This difference in design controls chromatic aberration. This does not correct the natural chromatic aberration of the eye, but it does correct any chromatic aberration introduced by the diffractive optic of the IOL (Figure 4). The expected benefit is an improvement of visual quality and high contrast sensitivity.
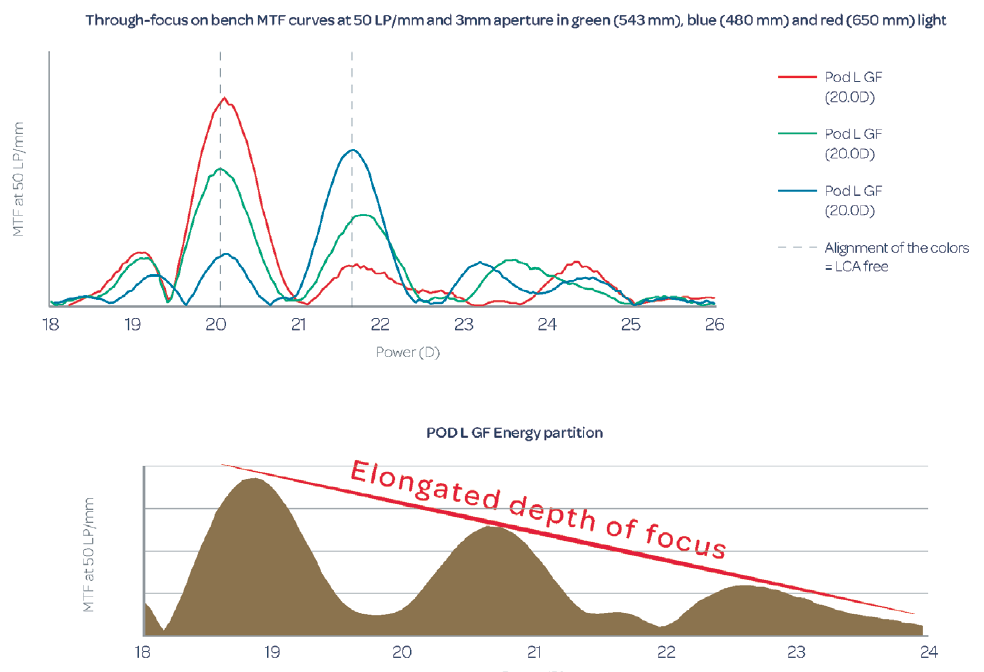
Figure 4. Through-focus on-bench modular transfer function curves at 50 lp/mm (data on file with PhysIOL).
Figures 3 and 4 courtesy of PhysIOL
IC-8
Small-aperture optics is an effective physiologic presbyopia-correcting solution for a range of accommodation loss, regardless of cataract status.
H. Burkhard Dick, MD, PHD, FEBOS-CR

The IC-8 IOL (AcuFocus; Figure 5) combines an aspheric monofocal IOL with an embedded opaque mini-ring to simultaneously address refractive error and presbyopia.
Implanted in the capsular bag at the time of cataract surgery (Figure 6), the one-piece hydrophobic acrylic IOL has modified C-loop haptics and an overall diameter of 12.5 mm. The biconvex aspheric optic is 6 mm in diameter with a square 360º posterior edge. The embedded annular mask has an outer diameter of 3.23 mm and a central aperture diameter of 1.36 mm.

Figure 5. The IC-8 IOL.
Courtesy of AcuFocus
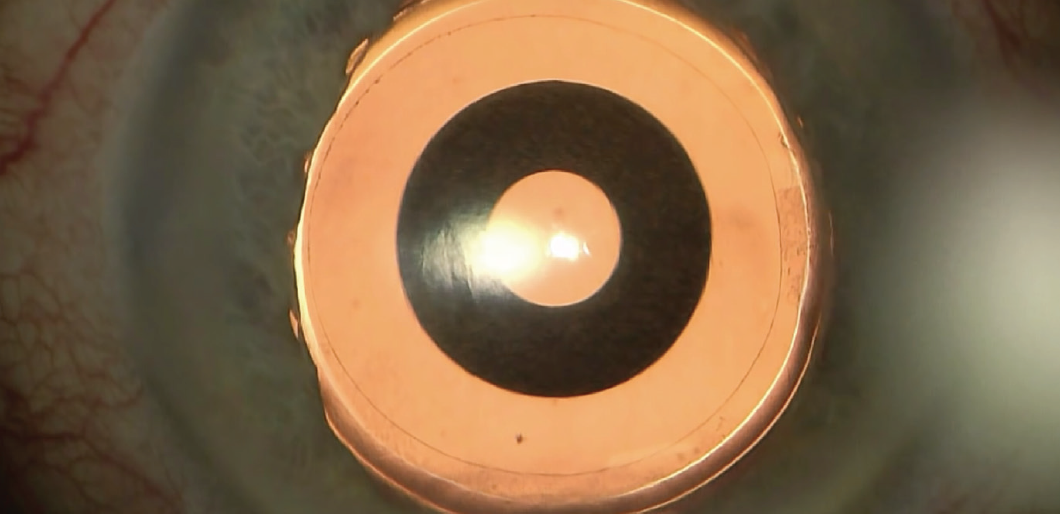
Figure 6. Intraoperative view of the IC-8 IOL after laser cataract surgery.
Courtesy of H. Burkhard Dick, MD, PhD, FEBOS-CR
availability
The IC-8 IOL has the CE Mark, and the company announced in June 2019 that enrollment for the pivotal US Investigational Device Exemption study had been completed. That 12-month prospective, multicenter, nonrandomized case control clinical trial is designed to evaluate the improvement in vision with the IC-8 IOL at all distances compared with traditional monofocal IOLs.1
Results from a multicenter postmarket European trial of 105 patients were recently published.2 Six months after implantation, monocular UDVA was 0.87 logMAR (20/23 Snellen), UIVA was 0.83 logMAR (20/24), and UNVA was 0.66 logMAR (20/30).
With respect to binocular vision, 99%, 95%, and 79% of patients achieved 20/32 or better UDVA, UIVA, and UNVA, respectively.2 The majority of patients (95.9%) reported that they would have the procedure again. Most (84.8%) reported using spectacles occasionally to never. Binocular contrast sensitivity matched the monocular contrast sensitivity achieved in the contralateral eye with a monofocal IOL.
Patients in this study and others have tolerated as much as 1.00 D of residual refractive error, suggesting that this small-aperture IOL may be more forgiving than monofocal and multifocal IOLs.2 Notably, astigmatic patients in a prospective clinical trial who received an IC-8 IOL and no additional astigmatic management were able to tolerate up to 1.50 D of refractive astigmatism.3
POTENTIAL PATIENTS
A capsular bag implant such as the IC-8 IOL is suitable for a broad range of patients, including those with irregular corneas.4,5 The implant can be used in patients with naturally occurring corneal higher-order aberrations of 0.6 µm or more or after corneal refractive surgery and keratoplasty.
Patients with diseased corneas, keratoconus, or ocular trauma are also potential candidates for this technology. The majority of patients do well with this lens, but some patients with larger mesopic pupils (≥ 6 mm) may experience photic phenomena due to light coming around the outside edge of the opaque ring.
As a result, it is important to evaluate pupil size when considering the IC-8 lens.
CONCLUSION
Small-aperture optics offer a dynamic, physiologic solution to the problem of presbyopia. The IC-8 IOL can be effective for a range of accommodation loss and for pseudophakia. As a whole, the category of small-aperture optics will continue to expand—an indication of the needs of our patients who live more active and longer lives.
1. AcuFocus completes study enrollment for U.S. IDE clinical trial of IC-8 lens. [press release]. Acufocus. June 14, 2019. http://www.acufocus.com/us/sites/default/files/MKU%20645%20Rev%20A%2C%20AcuFocus%20Completes%20Study%20Enrollment%20for%20U.S.%20IDE%20Clinical%20Trial%20of%20IC-8%20Lens.pdf. Accessed March 19, 2020.
2. Dick HB, Piovella M, Vukich J, et al. Prospective multicenter trial of a small-aperture intraocular lens in cataract surgery. J Cataract Refract Surg. 2017;43:956-968.
3. Ang RE. Small-aperture intraocular lens tolerance to induced astigmatism. Clin Ophthalmol. 2018;12:1659-1664.
4. Agarwal S, Thornell EM. Cataract surgery with a small-aperture intraocular lens after previous corneal refractive surgery: visual outcomes and spectacle independence. J Cataract Refract Surg. 2018;44(9):1150-1154.
5. Barnett V, Barsam A, Than J, Srinivasan S. Small-aperture intraocular lens combined with secondary piggyback intraocular lens during cataract surgery after previous radial keratotomy. J Cataract Refract Surg. 2018;44(8):1042-1045.
Juvene
This shape-changing two-part IOL is set to enter US clinical trials.
Uday Devgan, MD, FACS, FRCS

The Juvene (LensGen; Figure 7, inset) is a dual-optic, curvature-changing, modular IOL that provides a wide range of presbyopic correction. It has been in development for a decade, and I implanted the first batch in humans in initial clinical trials outside the United States late last year. This IOL is composed of two parts that are implanted and then coupled inside the eye.
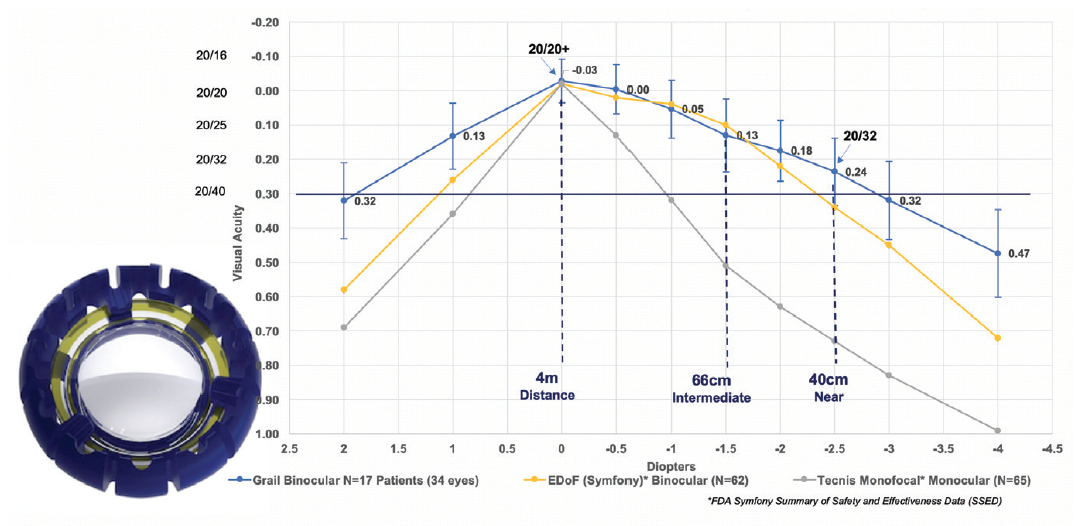
Figure 7. In a recent clinical study of the Juvene IOL (inset), the amplitude of accommodation provided by the IOL was about 3.00 D, with a range of good visual acuity from distance to 40 cm. The Juvene outperformed other lenses in range of vision and quality of vision (data on file with LensGen).
Figures 7 and 8 courtesy of Uday Devgan, MD
The promising results with the lens in those patients led us to start further studies in order to evolve the design of the Juvene IOL. In laboratory testing, the latest iteration of the Juvene lens shows 3.00 D or more of accommodative amplitude, which would theoretically provide a wide range of spectacle-free vision in patients. Our latest Juvene design was recently used in an exploratory clinical trial conducted outside the United States, named the Grail Study.
In the 17 patients (34 eyes) implanted in the Grail Study, binocular defocus curves showed a wide range of vision with both eyes corrected for plano distance (best DCNVA and distance-corrected intermediate visual acuity [DCIVA]), achieving a mean 20/25 intermediate vision at 60 cm and 20/32 near vision at 40 cm (Figure 7, line graph).1
Importantly, at all of these ranges of vision, the patient is provided with high-quality vision with normal contrast and no night glare or halos because there is no diffractive splitting of light with this lens. The patients were functionally spectacle-free and were pleased with their wide visual range and lack of dysphotopsias.
NO PCO
Over the past 5 years, the clinical investigators have collectively implanted more than 100 Juvene IOLs outside the United States, and one finding in particular has amazed us: No patients have had clinically significant posterior capsular opacification (PCO). We surmise that this is due to the nature of the Juvene, which completely fills the capsular bag and keeps the anterior and posterior capsular leaflets in their normal physiologic positions, preventing them from fusing.
We also carried out animal trials in a rabbit model that is commonly used to assess PCO (unpublished data). The Juvene IOL was placed in one eye and a control AcrySof one-piece acrylic IOL (Alcon) in the other eye. In assessments after 4 weeks, 6 months, and beyond, the AcrySof IOL showed a considerable fibrotic response with complete PCO, and the eyes with the Juvene IOL showed clear posterior capsules without significant fibrosis or PCO.
When these eyes were further examined with the Miyake-Apple view, we found that the Juvene IOL truly performed at an exceptional level with regard to biocompatibility and prevention of PCO (Figure 8).
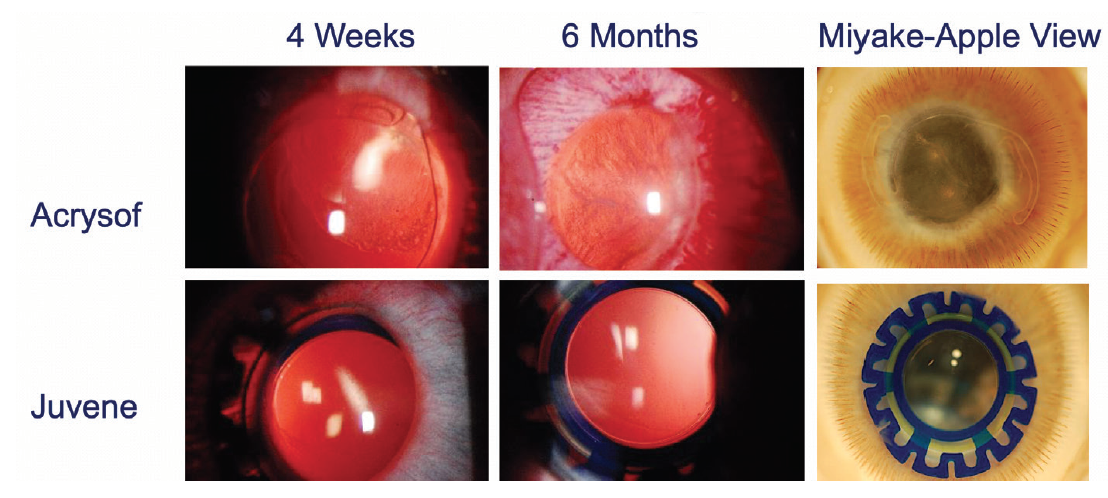
Figure 8. The Juvene IOL showed zero to minimal PCO in animal models meant to evoke a fibrotic response. The results in human studies over the past 5 years also confirmed the absence of clinically significant PCO.
THE FUTURE
We are now preparing to start US clinical trials to seek FDA approval for the Juvene. There will be multiple clinical sites across the country, and patient enrollment is expected to begin shortly. With a straightforward surgical technique, implantation through a standard-sized phaco incision, and 3.00 D of fluidic visual range without light-splitting, we anticipate a bright future for the Juvene IOL.
1. Garg S. Juvene IOL: Achieving patient expectations Paper presented at: Ophthalmology Innovations Summit. October 19, 2019; San Francisco.
enVista Trifocal
Currently, only one trifocal IOL is available in the United States; however, another trifocal design could hit the US market in the future: the enVista Trifocal (MX60EF, Bausch + Lomb). In its most recent press release on this technology, in 2018,1 Bausch + Lomb announced that it had enrolled the first patient in a clinical trial studying the efficacy and safety of the MX60EF to support FDA premarket approval for the lens.
The study is to include more than 500 patients undergoing bilateral cataract surgery. Patients will receive bilateral MX60EF trifocal IOLs or MX60E monofocal IOLs. Efficacy endpoints will be determined after 6 months and safety endpoints after 12 months.
The one-piece, UV-absorbing posterior chamber IOL will feature StableFlex technology for rapid optic recovery following delivery, AccuSet haptics with their offset design and broad contact angle, and the SureEdge design to provide a continuous 360º square edge, according to the company.
“The initiation of this trial is an important milestone for our organization and another example of our commitment to investing in research and innovation,” Chuck Hess, vice president and general manager, US Surgical, Bausch + Lomb, said in the press release.
1. Bausch + Lomb enrolls first patient in enVista MX60EF Trifocal IOL clinical trial [press release]. Bausch + Lomb. June 4, 2018. https://www.bausch.com/our-company/recent-news/artmid/11336/articleid/442/642018-monday/utm_campaign/september_conversion. Accessed March 19, 2020.
Omega Refractive Capsule
The flexible future: An artificial capsule to secure any IOL.
Gary Wörtz, MD

The human lens capsule is perhaps the most underappreciated anatomic structure in the eye. It is the thickest basement membrane in the body and has a successful track record of safely holding hundreds of millions of prosthetic implants (ie, IOLs) over the past several decades. It is devoid of innervation or blood supply, making it particularly inert and advantageous for this function. Unfortunately, the full potential of the capsule has never been realized.
Once an IOL is placed in the bag, surgeons hope never to have to remove it. But, according to a presentation made by Dean Corbett, BSc, MBChB, FRANZCO, at AECOS Aspen 2020,1 about one in 500 IOLs will require explantation due to a manufacturer issue (eg, calicification); patient intolerance of glare, halos, or dysphotospias; or improper power.
Given the tens of millions of cataract surgeries performed globally each year, it is time to start thinking about IOL implantation as more than a one-way trip. If surgeons can keep the capsular bag open and prevent capsular fibrosis, an almost unlimited number of options can be realized for helping patients in the early postoperative period and for years to come as their needs change or as lens technology improves.
A SOLUTION
The Omega Refractive Capsule (Omega Ophthalmics; Figure 9) is a device that solves this problem. This capsule has been 8 years in the making. Although its many prototypes evolved over the years, the concept has never wavered.

Figure 9. The Omega Refractive Capsule can be inserted through a 2.4-mm incision to create a stable environment inside the natural lens capsule.
Courtesy of Gary Wörtz, MD
The concept is this: The device is capable of holding any lens with a traditional C-arm haptic and preventing the capsule from collapsing and fibrosing around the lens or haptics. Not only does this allow easy exchange of any IOL, it also creates a measurable platform for lens position—a variable that has always been elusive to predict.
Beyond the lens and refractive implications, having a stable, open environment within the eye has potential for other therapeutic applications. Envision a day when surgeons have the opportunity to place an IOL, an IOP sensor, and sustained-released pharmaceuticals all within the safe confines of the Omega Refractive Capsule, free of current complications.
THE FULL RANGE
Cataract surgeons need a way to safely remove any lens implant without risking damage to the capsule or limiting the options for future IOL implantation. Omega Ophthalmics exists to provide an option to achieve this, finally addressing a problem that has plagued cataract surgery since the first lenses were implanted many decades ago.
Until this problem is solved, surgeons cannot provide the full range of desired services to their patients, which limits the adoption of advanced-technology presbyopia-correcting IOLs and, in the process, means some patients are unable to fully experience the joy they hoped for when they signed up for cataract surgery.
In its current design, the Omega Refractive Capsule can be inserted through a 2.4-mm incision to create a stable environment inside the natural lens capsule. For the near term, Omega plans to expand the testing of this device in multiple trials outside the United States and to move forward with a strategy to secure the CE Mark and FDA approval for the device as quickly as possible.
1. Corbett D. Cases: IOL exchanges. Paper presented at: American-European Congress of Ophthalmic Surgery; February 23-26, 2020; Aspen, Colorado.
SBL-3
Low levels of dysphotopsia are reported with this segmented bifocal lens.
Jeffrey C. Whitsett, MD

Over the past 3 years, I have been involved in the US phase 3 clinical trial of the SBL-3 IOL (Lenstec; Figure 10). The SBL-3 is an asymmetric segmented bifocal lens that provides a 3.00 D add with a transition zone connecting the distance and near segments. The lens is made in a biconvex design, with a 5.5-mm optic of hydrophilic acrylic material that is easily folded and inserted through a 2.4-mm incision.
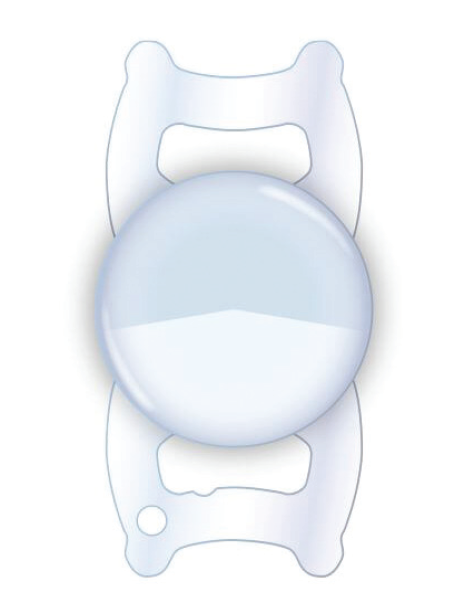
Figure 10. The SBL-3 has an asymmetric segmented bifocal lens design that provides a 3.00 D add with a transition zone connecting the distance and near segments.
Courtesy of Lenstec
The SBL-3 has been widely used outside the United States. Patients desiring a multifocal IOL for cataract surgery generally want high-quality vision, minimal unwanted side effects, and independence from glasses. The SBL-3 appears to meet these criteria and may have several design features that have led to its high patient satisfaction rate outside the United States.
CLINICAL EXPERIENCE
In my experience in the US clinical trial, the quality of distance vision has been excellent, although patients appear to prefer a slightly hyperopic target in order to obtain their best subjective result. In the study, we placed all the near segments inferonasally, but many international users place the segments in varied directions to maximize the function of the optic.
Placing the lens in a direction that allows the visual axis to be located within the distance segment of the optic appears to increase patient satisfaction and quality of vision. With concentric diffractive multifocal IOLs in patients with high angle kappa, we have found that one limitation is the difficulty of achieving centration on the visual axis. The SBL-3 may be an excellent option for patients with high angle kappa who desire a premium IOL.
In the course of the clinical trial, I have discussed the international experience with the SBL-3 with Mark Wevill, FCS(SA), FRCSEd, MBChB, of Optegra Eye Health Care in Birmingham, United Kingdom. He noted that his reasoning for choosing the SBL-3 centered on the high quality of vision with this IOL, the low number of night vision complaints, and the high level of independence from glasses that patients experience. Patients with occupational needs that include night driving or significant activity at night may particularly be good candidates for the SBL-3 because of this characteristic.
In our experience, night vision complaints with the SBL-3 are similar to those with a monofocal IOL. No patients in the study reported negative dysphotopsia, and patients reported a 93% rate of spectacle independence. Patients who required an occupational focus on near activity could address this with a pair of low-powered readers. Intermediate vision was excellent, with a notable range of focus.
LOOKING AHEAD
I believe that the SBL-3 and its sister lens, the lower add–power SBL-2, will be excellent options for refractive lens surgeon to offer their patients. Internationally, a combination approach—placement of the SBL-2 low-powered add in patients’ dominant eye and the SBL-3 higher-powered add in the fellow nondominant eye—has been successful (personal communication). Patients have reported high levels of satisfaction with minimal unwanted side effects.
There is no toric model of the SBL at present, so astigmatism must be corrected using limbal relaxing incisions. As with other multifocal IOLs, laser vision enhancement to eliminate residual refractive error is associated with significant improvement in patient satisfaction and IOL performance.
In March, Lenstec announced its submission of the SBL-3 IOL for premarket approval in the United States. I look forward to the FDA approval of the SBL-3 and believe the lens will be a welcome addition to offer patients seeking their best vision and independence from glasses after cataract surgery.
Tecnis Symfony Plus
An IOL with novel advances, including a violet light blocker.
Kerry K. Assil, MD

The Tecnis Symfony Plus (Johnson & Johnson Vision) is expected to become available to US surgeons by this summer. Modeled on the EDOF Tecnis Symfony (Figure 11), the Plus offers three advantages: (1) stronger near vision by about 1 line of near visual acuity, so that patients can read finer print, (2) a reduced level of dysphotopsia, and (3) the addition of a violet-light–blocking chromophore intended to compress chromatic aberration. The latter could possibly reduce dysphotopsia, improve contrast acuity, and provide further retinal protection.

Figure 11. The Tecnis Symfony IOL.
Courtesy of Johnson & Johnson Vision
BLOCKING VIOLET
All IOLs block harmful invisible UV light rays. Violet light is scattered more easily than light of other colors due to its short wavelength and high energy.1,2
Today, we are all exposed to more short-wavelength light due to high-efficiency light-emitting diodes (LEDs) in our smartphone screens, automobile headlights, and light bulbs. Because both violet and blue light fall on the short end of the visible light spectrum they are often grouped together, but there are important differences. Violet light (360 to 450 nm) has been associated with oxidative stress and retinal cell damage,3 whereas blue light (450 to 500 nm) has not been credibly associated with retinal damage and, in fact, has beneficial characteristics, including helping to regulate circadian rhythm and to preserve image quality in low light.4
A violet-blocking chromophore is already in use in IOLs available outside the United States. In a comparison with clear IOLs, significantly more patients with violet light–filtering IOLs experienced no difficulties driving in the daytime (P = .033) or at night (P = .017), and significantly more experienced no frustration with vision (89.8% vs 79.8%).5 Johnson & Johnson Vision researchers have found a 29% reduction in halo intensity from xenon headlights and a 13% reduction in halo intensity from smartphone LED light with these lenses compared to colorless IOLs.6 This validates the expectation that blocking short-wavelength violet light would result in better image quality.
CONCLUSION
At present, the Tecnis Symfony lens is the only presbyopia-correcting IOL on the market that approaches the distance clarity and contrast acuity of a monofocal IOL. How the Symfony Plus lens fits into our armamentarium of presbyopia-correcting IOLs will depend on the near and distance vision results we obtain clinically. When I had the opportunity to evaluate the Tecnis Symfony Plus in a multicenter study, I found that the improvement in near vision compared with the Symfony was large, and any reduction in distance acuity, if present, appeared to be subclinical. My patients noted fewer dysphotopsias. In my early experience, patient satisfaction with the lens was extremely high.
Time will tell whether Tecnis Symfony Plus achieves the same distance acuity benchmark and thus replaces Tecnis Symfony in our practice or whether the two end up being complementary, either in a personalized mix-and-match scenario or through patient selection.
1. University of Arizona College of Optical Sciences (2013) Ocular scatter. https://wp.optics.arizona.edu/visualopticslab/wpcontent/uploads/sites/52/2016/08/Class10_11.pdf
2. Tosini G, Ferguson I, Tsubota K. Effects of blue light on the circadian system and eye physiology. Mol Vis. 2016;22:61-72.
3. Marie M, Bigot K, Angebault C, et al. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018;9(3):287.
4. Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol. 2006;90(6):784-792.
5. Canovas C, Weeber H, Trentacost D, et al. Optical and visual performance of violet blocking intraocular lenses. Poster presented at: Association for Research in Vision and Ophthalmology Annual Meeting; April 28-May 2, 2019; Vancouver, Canada.
6. Data on file with Johnson & Johnson Vision.
Light Adjustable Lens: EDOF Adjustability in the Pipeline
In the FDA clinical trial of the Light Adjustable Lens (RxSight), investigators reported excellent results with this first-of-its-kind IOL, including reduction in treated astigmatism, excellent UCVA, and a sharp reduction in residual spherical error.1 A little less than 2 years after FDA approval, the company announced the initial commercialization of the monofocal Light Adjustable Lens in the United States,2 and surgeons have continued to report excellent outcomes (see “Newcomers”).
As currently approved, the Light Adjustable Lens can be treated postoperatively with the Light Delivery Device (RxSight) to adjust sphere and cylinder. Now, US surgeons and patients hope to gain access to an extended depth of focus (EDOF) treatment for the Light Adjustable Lens that is already approved in Europe and Mexico, according to Market Scope.2
RxSight has demonstrated the EDOF adjustability in 18 patients who underwent bilateral implantation of the Light Adjustable Lens outside the United States. All were given EDOF adjustments, and all achieved uncorrected distance and intermediate visual acuities of 20/20 or better and uncorrected near visual acuity of 20/25 or better.2 Market Scope reported that a US trial under an investigational device exemption is under way.
1. FDA approves first implanted lens that can be adjusted after cataract surgery to improve vision without eyeglasses in some patients [press release]. US Food and Drug Administration. November 22, 2017. https://www.fda.gov/news-events/press-announcements/fda-approves-first-implanted-lens-can-be-adjusted-after-cataract-surgery-improve-vision-without. Accessed March 19, 2020.
2. RxSight begins initial commercialization of Light Adjustable Lens in the US. Market Scope Ophthalmic Market Perspectives. September 26, 2019. https://www.rxsight.com/media/rjrbad2g/marketscope_2019_sep_26.pdf. Accessed March 19, 2020.
Tecnis Synergy
The EDOF addition improves near vision without sacrificing distance vision.
Frank T. Kerkhoff, MD, PhD

The Tecnis Synergy IOL (Johnson & Johnson Vision; Figure 12) was introduced in Europe last year, and since that time I have successfully implanted about 300 of them. Two of my senior optometrists, who have decades of experience and have been involved with more than 10,000 presbyopia-correcting IOL cases each, have chosen bilateral Tecnis Synergy lenses for their own eyes, which I think is a testament to the excellence of this lens.

Figure 12. The Tecnis Synergy IOL.
Figures 12 and 13 courtesy of Johnson & Johnson Vision
The Synergy combines diffractive multifocal optics with an EDOF addition at near. It also filters ultraviolet and violet light, but it does not filter blue light. I consider it to be most similar to a trifocal lens. Compared with trifocal lenses, the Tecnis Synergy has a more natural range of near to intermediate vision and better mesopic near vision (Figure 13).
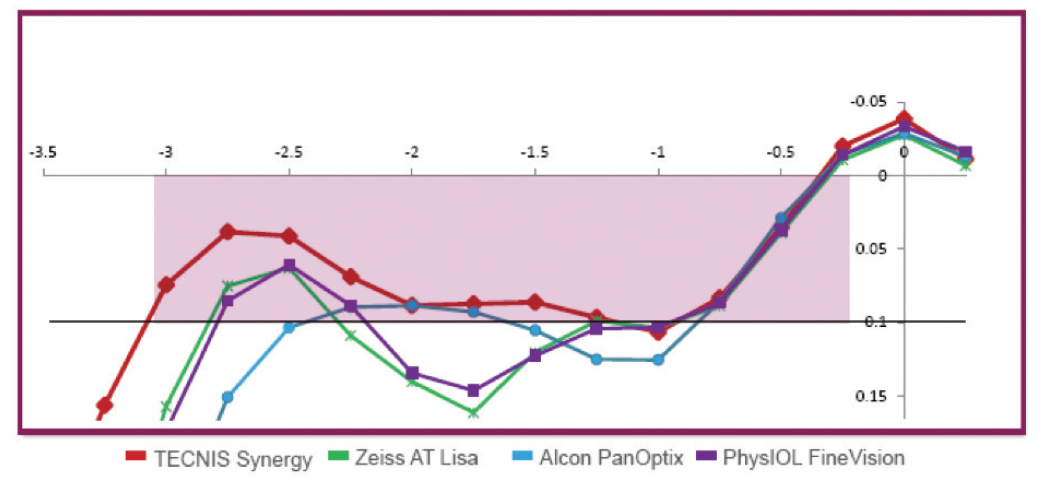
Figure 13. The Tecnis Synergy IOL offers a natural, continuous range of vision from near through intermediate, as well as high-quality distance vision (data on file with Johnson & Johnson Vision).
NEAR GAINS
The near point of the Synergy is a little closer than the near point of trifocal IOLs, at about 35 cm. From the near point through about 70 cm, the lens’ diffractive echelette optics provide a continuous range of near to intermediate vision that patients say feels natural. A range like this is ideal for working-age patients who wish to be able to look from their desk to their computer to a colleague a few feet away, or for those who are shopping and cooking and need to be able to see items on the grocery shelf or in their kitchen without constantly adjusting how far away they are from the objects.
The Synergy is relatively pupil independent, and in my experience, patients report that they can still read in dim light. In fact, I have heard no complaints about near vision at all.
DISTANCE AND NIGHT VISION
The near-vision gains associated with the Synergy IOL have been achieved without sacrificing the excellent quality of distance vision that we have come to expect from the Tecnis platform. Like the other lenses in this IOL family, the material induces minimal chromatic aberration, and this is further reduced by the optic design, especially in dim light. When the pupil expands, the diffractive pattern corrects even more chromatic aberration, which explains the high-quality vision patients report in low light situations. I have also found that the lens reduces spherical aberration to near zero in most eyes.
Of course, because it has diffractive optics, the Synergy lens still has some issues with glare and halos at night—but these photic phenomena are generally well tolerated. My patients report that they are an acceptable trade-off for the quality and range of vision.
NO TORIC YET
The only other disadvantage of this lens is that it is currently not available in a toric version. In our refractive clinic, more than half of patients need correction of astigmatism, so this limits the number of patients who are good candidates for the Synergy lens. If a toric option becomes available, the Synergy will certainly be the most frequently implanted presbyopia-correcting IOL in my clinic.
xact Mono-EDoF
This lens acts like a true monofocal while providing a continuous focus from distance to intermediate.
Mark Packer, MD, FACS, CPI

The ongoing quest for the perfect presbyopia-correcting IOL has led to a series of compromises involving the trade-offs of multifocal dysphotopsia and reduced contrast sensitivity in exchange for spectacle independence. Although the benefits outweigh the risks for many patients, the relatively high cost of premium IOLs can exacerbate some patients’ dissatisfaction and lead to secondary surgery and IOL explantation.1
A new entry in the field of presbyopia correction seeks to minimize risk by delivering quality of vision and contrast sensitivity comparable to those of a monofocal IOL while providing an extended depth of focus with very low incidence of halo and glare compared with other diffractive EDOF lenses.
The xact Mono-EDOF IOL (Santen), a one-piece lens manufactured from a glistening-free hydrophobic acrylic material, meets international bench testing criteria for a monofocal IOL and has received the CE Mark as a monofocal IOL. Its optic, which incorporates four diffractive rings (Figure 14), demonstrates a single, broad through-focus modulation transfer function (MTF) peak. In comparison, traditional EDOF IOLs exhibit dual peaks.2 MTF measurements and retinal image modeling show that the xact IOL has a higher tolerance to tilt and decentration than other EDOF IOLs with at least 40% higher MTF. This directly affects the optical quality of the image delivered to the retina.3

Figure 14. The xact Mono-EDOF IOL.
CLINICAL RESULTS
Clinical trials with the xact Mono-EDOF IOL have taken place at several sites in Europe, including clinics in Germany, the United Kingdom, France, and Portugal. The largest body of data to date has been provided by the International Vision Correction Research Centre at the University of Heidelberg, Germany.
In the Heidelberg study of the xact IOL, for which I am the medical monitor, 44 eyes of 25 patients (10 men and 15 women) have been followed for 9 months.4 The mean age of patients is 70.3 years, and their mean preoperative monocular BCVA was 0.33 ±0.16 logMAR (equivalent to 20/40-).
At 9 months, mean monocular BCVA improved to -0.05 ±0.13 logMAR (20/20+), and mean monocular UCVA was 0.05 ±0.19 logMAR (20/20-). Monocular DCIVA at 66 cm was 0.21 ±0.11 logMAR (20/30-), and monocular UIVA at 66 cm was 0.18 ±0.13 logMAR (20/30+).
With binocular testing, DCIVA was logMAR 0.09 ±0.13, and UIVA was logMAR 0.08 ±0.10 (20/25+).
Monocular and binocular defocus curves are shown in Figure 15. Binocular depth of focus at 20/30 is estimated to be 1.30 D. Of note, the defocus peak demonstrates a broad area within the range of ±0.50 D where visual acuity is better than 20/20, suggesting a relatively forgiving tolerance to normal variations in the accuracy of IOL power calculation. One would therefore expect binocular UDVA of 20/20 or better within the dioptric range of ±0.50 D postoperative manifest refraction spherical equivalent.
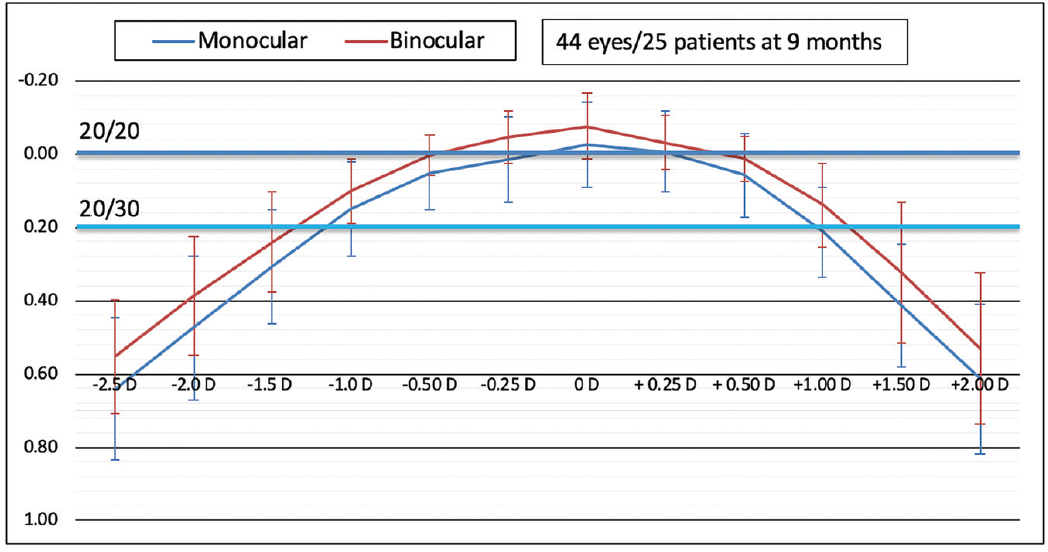
Figure 15. Defocus curves demonstrate 1.30 D binocular defocus at 20/30 and a broad peak within the range of ±0.50 D postoperative manifest refraction spherical equivalent (data on file with Santen).
Figures 14 and 15 courtesy of Santen
Testing with the Halo and Glare Simulator (Eyeland Design Network) demonstrated only slight interference from dysphotopsia. Approximately 75% of patients reported little difficulty driving at night. Contrast sensitivity measured within the expected range for monofocal IOLs.
CONCLUSION
The xact Mono-EDOF IOL is an exciting entry in the field of presbyopia-correcting IOLs, providing quality of vision similar to that of a monofocal IOL with vision in the intermediate range like that of an EDOF IOL. Early clinical results suggest that this lens will be a valuable addition to cataract surgeons’ repertoire of lenses.
1. Packer M. Enhancements after premium IOL cataract surgery: tips, tricks, and outcomes. In Starr C, ed. Current Ophthalmology Reports. New York: Springer Science + Business Media; 2013.
2. Spalton DJ, Packer M, So Y, Tiwari N, Venkateswaran K. Optical and clinical comparison of a novel monofocal extended-depth-of-focus IOL and a conventional bifocal extended-depth-of-focus IOL. Paper presented at: ASCRS Annual Meeting; May 4, 2019; San Diego, CA.
3. Spalton DJ, So Y, Tiwari N, Venkateswaran K. The effect of tilt and decentration on a novel extended-depth-of-focus IOL compared to conventional extended-depth-of-focus IOLs. Paper presented at: ASCRS Annual Meeting; May 4, 2019; San Diego, CA.
4. Weindler J, Baur I, Son HS, et al. Prospektive klinische Studie mit einer neuen monofokalen Intraokularlinse mit erweiterter Tiefenschärfe. Paper presented at: Kongress der DGII; February 13, 2020; Mainz, Germany.




