Managing a Malpositioned Toric Lens
A step-by-step guide to addressing an unwelcome outcome.
Brandon J. Baartman, MD

Minimizing astigmatism at the time of lens replacement is one of the most crucial determinants of patient satisfaction after refractive cataract surgery. This can be accomplished by placement of a toric IOL, aiming to neutralize the patient’s natural corneal astigmatism.
Success with these lenses requires that many pre- and intraoperative criteria be met. The patient’s eye must have a healthy tear film and ocular surface, and the surgeon must obtain reliable corneal topography and keratometry, achieve proper corneal axis marking or intraoperative aberrometry reading, accurately estimate surgically induced astigmatism, and precisely position the toric lens. Even when all of these benchmarks have been accomplished, success is predicated on the rotational stability of the lens.
Although, in general, modern toric IOLs have excellent rotational stability, unintended lens rotation is still something refractive cataract surgeons must be aware of and, if it happens, have a plan for addressing. This article lays out some pointers for successful management of a malpositioned or rotated toric IOL.
EVALUATION OF THE ROTATED LENS
Appropriate management of a malpositioned toric IOL ultimately depends on the patient’s symptoms, residual refractive error, and goals. Looked at in this way, the postoperative care of these patients actually begins before surgery, during the discussion of goals and possible outcomes.
If the patient’s goal is to reduce (but not necessarily to eliminate) spectacle use, he or she may be satisfied with a prescription pair of glasses for a small residual refractive error. Most patients, however, are more discerning and have elected a toric lens desiring a spectacle-free outcome. For these patients, a refractive adjustment is necessary, and arriving at an appropriate strategy for that adjustment takes some consideration. Generally, the solution is either to rotate the lens or to leave it as is and perform an enhancement in the form of corneal refractive surgery to reduce the residual error.
The first step in addressing the error is to perform a precision refraction at each postoperative follow-up visit, including day 1. A careful refraction at the phoropter will provide the most accurate representation of the magnitude of the problem, and any reduction in BCVA must be explained to make sure an accurate refraction has been obtained. It’s also important to determine the presence of spherical refractive error, which may suggest that an IOL exchange for a different spherical and/or toric power is warranted. Review of the patient’s preoperative astigmatism and of the cylinder power of the toric IOL is important, as small rotations are more significant with higher-power toric lenses.
Close inspection of the ocular surface to identify dryness—not uncommon after cataract surgery—is also important to ensure that this is not a factor. Dilated inspection of the lens markings, the position of the haptics, capsule-optic overlap, lens tilt, and posterior capsular distension or opacification is crucial in determining the cause of the residual refractive error.
Once an in-the-bag and planar position of the lens has been confirmed, the position of the lens markings should be documented and compared with the intended placement. This can be done at most slit lamps via rotation of a fine slit beam. Another alternative is to use any number of smartphone applications that work using the phone’s camera and accelerometer to note the rotation of the phone itself when it is aligned with the lens.
SECONDARY INTERVENTION
A malpositioned toric IOL may be able to be rotated into a position that minimizes refractive cylinder. The manifest refractive error, along with lens power details and position, can be used with an online toric back-calculator, such as Berdahl and Hardten’s Toric Results Analyzer, to determine whether and by how much astigmatism can be reduced by rotating the lens (Figure 1).
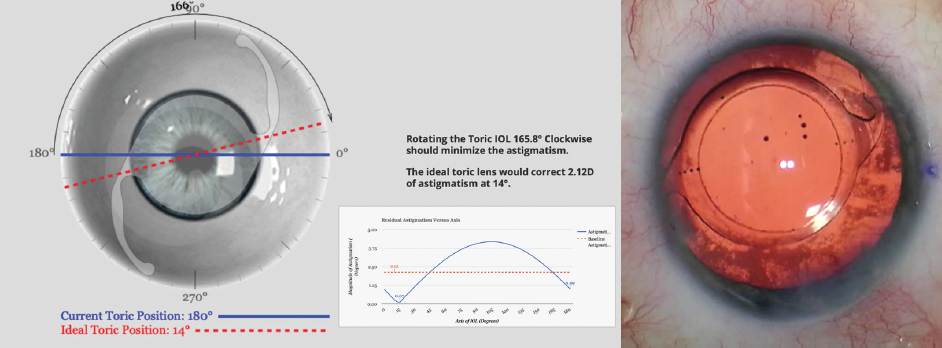
Figure 1. Screenshot from toric back-calculation for a patient with astigmatism after toric IOL placement (left). Rotation of the lens as prescribed in the clockwise direction can significantly reduce residual astigmatism in this case. OR microscope photo (right) demonstrates the lens in this patient after secondary rotation.
Courtesy of Brandon J. Baartman, MD
Generally, this approach can be considered in the following situations:
- If the malposition is noted early in the postoperative period;
- In eyes with significant residual astigmatism; and
- At any point in the postoperative period in an eye not otherwise well suited for corneal refractive surgery.
Lens rotation is typically performed in the OR under sterile conditions, although some have advocated for office-based surgery. The risks of the lens rotation procedure are generally the same as those of the original surgery. Aberrometry or limbal marks may be used to assist in accurate lens rotation. In these scenarios, I often rely more heavily on limbal markings of the current and intended positions rather than intraoperative aberrometry.
The suspected cause of the rotation should be considered when a repeat trip to the OR is planned. For instance, if the cause was the presence of residual OVD behind the lens or a large axial length, these factors may allow the lens to rotate again if not addressed. A capsular tension ring (CTR) can be placed at the time of primary lens placement or during a secondary rotation in eyes with large axial lengths in an attempt to improve rotational stability.
If the lens malpositioning has resulted in a small amount of residual astigmatism, early observation may be warranted. Subtle shifts in refraction from wound healing, ocular surface changes, or capsular contraction may minimize symptoms if given the time to resolve.
For patients with symptomatic refractive error, corneal refractive surgery can be performed at 3 months postoperative when refraction is stable. LASIK and surface ablation are two commonly employed techniques for enhancement. Either technique is likely an accurate way to reduce residual refractive error and offers the ability to correct residual spherical error as well as cylinder.
If the spherical equivalent is close to plano, the surgeon can also consider using corneal relaxing incisions. A number of nomograms are available to help guide treatment planning.
CONCLUSION
Having a plan for addressing unintended lens rotation of a toric IOL helps to ensure that even those patients who are initially unhappy with their visual results can achieve the best possible outcomes postoperatively. Starting your planning with adequate discussion of goals and possible outcomes of astigmatism correction ensures that patients are aware of the risk of lens rotation. Then, if malpositioning occurs, the surgeon can decide between rotating the lens or leaving it as is and performing a corneal refractive surgery enhancement to reduce the residual error.
Management of Dislocated Presbyopia-Correcting IOLs
Strategies may be chosen based on the particulars of the case.
Alanna Nattis, DO, FAAO

As innovation surrounding cataract and refractive surgery evolves, we are afforded the opportunity not only to improve but also to enhance our patients’ vision. The results achieved with presbyopia-correcting IOLs have steadily improved over time, and at the same time potential side effects have decreased.
That being said, most cataract surgeons understand that not every patient is an ideal candidate for a presbyopia-correcting IOL.1,2 Certain personality traits, career profiles, and anatomic findings can predispose patients to a less-than-successful surgical result.1 Screening patients carefully for findings such as zonular laxity and phacodonesis and providing comprehensive explanations of the risks, benefits, and alternatives associated with presbyopia-correcting IOLs can help surgeons to produce happy postoperative patients.
Still, there will be cases—fortunately not commonly—when a patient may experience dislocation of a presbyopia-correcting IOL.2-6 Early causes of dislocation include complications during surgery (eg, vitreous loss, zonular loss, zonular damage) and IOL malpositioning. Late causes of dislocation can include trauma, pseudoexfoliation, capsular contraction, and subsequent posterior segment surgical procedures. There have been case reports of IOL decentration after intravitreal injection, and IOL tilt can occur after vitrectomy as well.6
HAVE A PLAN
Because centration is crucial to the performance of presbyopia-correcting IOLs, it is important not only to recognize decentration when it happens but also to have a plan for management.2-5,7,8 It is also crucial to check for signs of decentration before performing Nd:YAG capsulotomy; the posterior capsular opening can make subsequent repositioning or exchange surgeries more difficult.2
Management strategies for a decentered presbyopia-correcting IOL can be chosen based on the severity, location, and nature of the decentration. Mild decentration can be managed by returning to the OR, meticulously polishing the capsule to remove lens epithelial cells, and placing a CTR. All of these can be done through a small incision.5,8
When a CTR is used, it can be placed into the capsular bag at any time after creation of the capsulorhexis, generally at the first sign of zonular weakness. In most cases, the CTR will provide satisfactory stability, but in severe instances the capsule may remain loose or decentered. In this event, a capsular tension segment with or without scleral fixation of the capsular bag–IOL complex may be necessary.
If the IOL is partially subluxed out of the bag (eg, one haptic in and one out, one haptic in and both the optic and the other haptic out), surgical repositioning is required. One method is to viscodissect the bag open using a cannula or bent 30-gauge needle attached to a vial of dispersive OVD. Once the bag is open, a cannula or Sinskey hook can be used to gently rotate and center the IOL into the appropriate position.3,8
Anterior capsular contraction can cause the IOL to shift, creating astigmatism and a change in vision.2,3,8 With capsular phimosis and a small rhexis, the IOL can not only tilt, causing astigmatism, but can move posteriorly in the eye, inducing hyperopia. Management can include Nd:YAG laser lysis of the anterior capsular rim to release contracture, which may return the IOL to the correct position.2,8
If the decentered lens is in the sulcus and the capsular bag is compromised, the lens can be fixated to the iris or sclera. Multiple techniques to accomplish this goal have been described, including the commonly used modified Siepser9 and McCannel10 techniques. The glued IOL11 and the Yamane intrascleral haptic fixation12 (Figure 2) techniques are excellent options for scleral fixation and centration of three-piece IOLs.
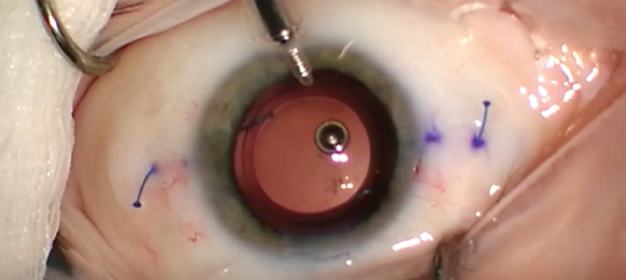
Figure 2. The Yamane technique obviates the need for glue or sutures.
Courtesy of Kamran Riaz, MD
All iris and scleral fixation methods require care. Because they involve haptic manipulation, it is important to consider the type of IOL and the length of time it has been in the eye. A haptic breaking during manipulation for iris or scleral fixation can turn a relatively straightforward procedure into a complex, much longer surgery and even risk loss of the IOL into the posterior segment, among other complications.
Finally, if there has been a complete dislocation of the IOL into the vitreous cavity or onto the retina, assistance from a retinal surgeon is necessary to bring the IOL anterior for fixation or removal. Depending on the type of IOL, it may have to be removed and exchanged. A one-piece IOL cannot be fixated to the sclera, nor should it be placed in the sulcus due to risk of uveitis-glaucoma-hyphema syndrome, so it must be swapped for a three-piece or anterior chamber IOL.
COMFORT ZONE
For any of the scenarios described here, the surgeon should use whichever technique he or she is comfortable with. If sufficient previous experience is lacking, it is helpful to review a novel technique in person with another experienced surgeon before attempting it oneself. Performing advanced, complex surgery with an experienced assistant is priceless, and his or her presence can help save the day.
Prevention is always the optimal strategy to address decentration. In high-risk patients—those with severe pseudoexfoliation, history of trauma, or phacodonesis, for example—the consent process must include extensive discussion of the risks of surgery. Two important risks to include are the possibility that it will be impossible to implant the chosen IOL and that late complications include dislocation and its sequelae. Often, patients are happy that their surgeon has spent the chair time discussing their unique conditions with them, and they will be highly satisfied with any lens decided upon.
1. Rosenberg E, Nattis A, Donnenfeld ED, Barsam A. Refractive intraocular lenses – managing unhappy patients. In: Hovanesian J, ed. Refractive Cataract Surgery: Best Practices and Advanced Technology. Thorofare, NJ: Slack; 2017: 215-224.
2. Schena LB. Centration – the heart of IOL success. EyeNet Magazine. January 2007.
3. Decentered multifocal IOLs may be cause of worsened vision. Ocular Surgery News. April 10, 2015.
4. Packer M, Fine IH, Hoffman RS, Piers PA. Improved functional vision with a modified prolate intraocular lens. J Cataract Refract Surg. 2004;30(5):986-992.
5. Caceres V. Dislocation and decentration remain common reasons for IOL removal. Ophthalmology Times. August 8, 2019.
6. Degenring RF, Sauder G. Vitreous prolapse and IOL dislocation during intravitreal injection of triamcinolone acetonide. Graefes Arch Clin Exp Ophthmol. 2006;244(8):1043-1044.
7. Jafarinasab M, Feizi S, Baghi A, Ziaie H, Yaseri M. Aspheric versus spherical posterior chamber intraocular lenses. J Ophthalmic Vis Res. 2010;5(4):217-222.
8. Stephenson M. How to manage dislocated IOLs. Review of Ophthalmology. October 14, 2018.
9. Osher RH, Snyder ME, Cionni RJ. Modification of the Siepser slip-knot technique. J Cataract Refract Surg. 2005;31(6):1098-1100.
10. Wald KJ, Paccione JC, Gross NE, et al. Successful management of posteriorly dislocated PC-IOLs with the twenty-five gauge vitrectomy and modified McCannel suture technique. Invest Ophthalmol Vis Sci. 2007;48(13):2207.
11. Narang P, Agarwal A. Glued intrascleral haptic fixation of an intraocular lens. Indian J Ophthalmol. 2017;65(12):1370-1380.
12. Yamane S, Sato S, Maruyama-Inoue M, Kadonosono K. Flanged intrascleral intraocular lens fixation with double-needle technique. Ophthalmology. 2017;124(8):1136-1142.




