

The algorithm of glaucoma treatment has shifted dramatically, as a growing number of ophthalmologists have begun offering microinvasive glaucoma surgery (MIGS). The safety and effectiveness of these procedures have made them an attractive option for early and moderate disease. As defined by Saheb and Ahmed, MIGS uses an ab interno microincision, produces minimal trauma, has a high safety profile, and is followed by rapid recovery.1 This article reviews current MIGS options in the United States and previews the devices that may become available here in the future.
CURRENTLY AVAILABLE
Trabectome
Introduced in 2004, the Trabectome (NeoMedix) allows surgeons to perform a trabeculotomy via an internal approach. The device uses electrocautery to remove a strip of the trabecular meshwork and unroof Schlemm canal to allow aqueous to flow freely out of the eye. Surgeons can treat approximately 120º through a single incision, although larger treatment areas can be achieved with additional incisions.
iStent
The iStent Trabecular Micro-Bypass Stent (Glaukos) was approved in 2012. This heparin-coated, nonferromagnetic titanium stent is shaped like a snorkel to facilitate implantation. At 1 mm in length, the iStent is currently the smallest FDA-approved device. It can be inserted at the time of cataract surgery through an incision of 1.5 mm or larger. The current FDA approval allows for the placement of just one iStent at the time of cataract surgery, but studies are underway on the implantation of additional stents as well as on the device’s use in patients who are pseudophakic.
Gonioscopy-Assisted Transluminal Trabeculotomy
Gonioscopy-assisted transluminal trabeculotomy is an ab interno procedure that involves creating a goniotomy, cannulating Schlemm canal by passing a suture or a microcatheter through 360º of the canal, and retrieving the distal end of the suture or microcatheter, which the surgeon externalizes to complete the 360º trabeculotomy. Doing so removes any trabecular meshwork tissue that is contributing to outflow resistance.
Ab Interno Canaloplasty
This procedure is similar to gonioscopy-assisted transluminal trabeculotomy in that a goniotomy is performed and Schlemm canal is cannulated by a microcatheter, but viscodilation is also performed. Once the microcatheter has passed through 360º of the canal, it is gently withdrawn along the same pathway, and viscoelastic is injected to dilate Schlemm canal and the collector channel system to restore aqueous flow of the distal drainage system.
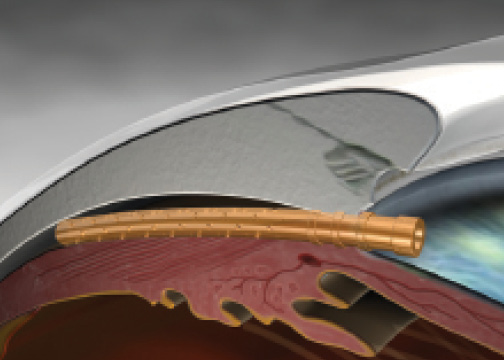
Figure 1. The CyPass Micro-Stent implanted.
FUTURE DEVICES
Trabecular Meshwork
It is easiest to classify the devices in development based on their anatomical placement. The second generation of the iStent, the iStent Inject (Glaukos), is currently in phase 3 FDA trials. Like its predecessor, the iStent Inject is also composed of titanium, but it is only 360 µm long. It also has a different shape. The stent has a narrow lumen and an apical head with four inlets through which aqueous can flow. A flange base secures the stent and allows aqueous to pass into Schlemm canal. The surgical technique is slightly easier for the iStent Inject than the iStent, because there is no sideways sliding of the former. Moreover, the iStent Inject has two stents preloaded, so surgeons can place both without exiting the eye.
The Hydrus (Ivantis) offers a dual-mechanism approach. This 8-mm flexible device is composed of nitinol. Like the iStent, the Hydrus has a snorkel that allows aqueous to flow into Schlemm canal. The length of the device allows it to dilate and support Schlemm canal for approximately 3 clock hours. The dilation of the canal is thought to allow greater flow of aqueous through the trabecular meshwork, because it places the trabecular meshwork on tension. The collector channels are segmented, and the Hydrus disrupts the tissue bridges, allowing aqueous to flow through the device and access more collector channels. Enrollment is complete in the Hydrus IV pivotal trial. (For more on this trial and the Hydrus, visit eyetube.net/?v=iluli.)
The design of the Kahook Dual Blade (New World Medical) is somewhat similar to that of the Trabectome, but the former uses actual blades instead of electrocautery. The tapered tip of the Kahook Dual Blade is designed to ease the instrument’s entry into Schlemm canal, after which the device slides along the trabecular meshwork. The blade that is in the canal lifts and stretches the trabecular meshwork so that the second blade can safely cut the tissue. This allows for cleaner tissue removal and minimizes damage to adjacent tissue.2
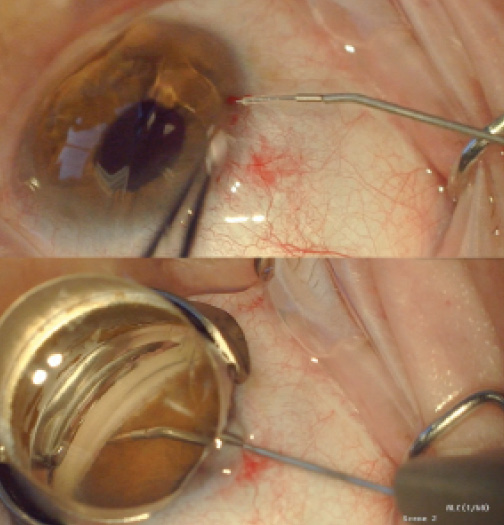
Figure 2. The iStent Supra attached to the inserter prior to entry into the eye (A) and after full insertion into the suprachoroidal space (B).
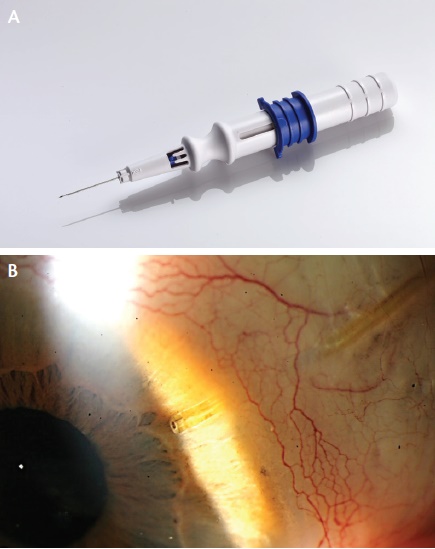
Figure 3. The Xen45 is implanted via a 27-gauge needle (A). View of the shunt at the slit lamp (B).
Suprachoroidal Space
The CyPass Micro-Stent (Transcend Medical) is made of a polyimide material. The device is a tube that is 6 mm long and has an inner diameter of 300 µm that allows aqueous to flow from the anterior chamber to the suprachoroidal space. The surgeon places the stent under gonioscopy, with the target located between the scleral spur and the ciliary body. The stent then enters the potential space between the inner scleral wall and the ciliary body and choroid (Figure 1). Transcend Medical announced completion of the Compass pivotal trial evaluating the CyPass Micro-Stent, and the company expects to file a premarket approval application with the FDA in the second half of this year.
Like the CyPass, the iStent Supra (Glaukos) targets the uveoscleral outflow pathway (Figure 2). A US investigational device exemption trial of the stent is underway.
Subconjunctiva
The Xen45 (AqueSys) is a soft, tubular implant composed of porcine gelatin that is cross-linked with gluteraldehyde. The device is 6 mm long and has three different lumen sizes of 45, 63, and 140 µm. The flexible shunt is located within a 27-gauge needle, which is passed through the anterior trabecular meshwork and sclera but under the conjunctiva, allowing the tubular structure to deliver aqueous from the anterior chamber to the subconjunctival space (Figure 3). A bleb then forms, without any external cutting or suturing. Despite the formation of a bleb, the Xen45 still seems to fit the definition of MIGS. The US investigational device exemption trial of the Xen45 is fully enrolled. Allergan recently announced that it will acquire AqueSys and that it expects final FDA approval of the technology via the 510(k) device pathway in 2016 or early 2017.
With multiple devices addressing various mechanisms on the way, the outlook for MIGS is promising.
1. Saheb H, Ahmed IIK. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96-104.
2. Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013;155(3):524-529.
Michael Greenwood, MD
• fellow, glaucoma, cornea, cataract, and refractive surgery, Vance Thompson Vision, Sioux Falls, South Dakota
• (605) 361-3937; migreenw@gmail.com; Twitter: @migreenw
• financial interest: none acknowledged
John Berdahl, MD
• clinician and researcher, Vance Thompson Vision, Sioux Falls, South Dakota
• (605) 361-3937; john.berdahl@vancethompsonvision.com
• financial disclosure: consultant to Glaukos


