
The FDA approved the femtosecond laser for cataract surgery in 2010, specifically to correct astigmatism. As surgeons gain experience with this technology, however, they are finding it offers other advantages. Many patients with coexisting glaucoma and cataract have narrow angles, pseudoexfoliation, Fuchs dystrophy, pupils that do not dilate well, or other abnormalities. In these cases, using a femtosecond laser to perform part of the cataract procedure can be extremely helpful.
Whether the laser’s use is based on specific pathology or not, however, it generally represents an out-of-pocket cost to the patient, whose demands and expectations then increase. When a patient has coexisting cataract and glaucoma, the surgeon faces the challenge of addressing IOP at the time of surgery in a way that does not compromise refractive results or require a postoperative regimen involving multiple hypotensive drops. An effective option is endoscopic cyclophotocoagulation (ECP).
RESEARCH
Although traditional filtering surgeries are highly effective at lowering IOP, they also tend to increase astigmatism.1 ECP is unique among surgical glaucoma treatments for its versatility. Because the procedure targets aqueous production rather than outflow, ECP is useful for open-angle as well as angle-closure glaucoma, and it has applications regardless of disease severity. Although some previous studies reported that ECP affected cataract surgery outcomes,2 my fellow investigators and I found that ECP combined with phacoemulsification effectively lowered IOP without significantly affecting visual acuity or postoperative complications.3 Inflammation is often higher after the combined procedure than after laser cataract surgery alone. I have found proactive management with a transzonular injection of TriMoxi (Imprimis Pharmaceuticals) at the conclusion of surgery effective. I find that eyes are in an excellent condition from day 1.
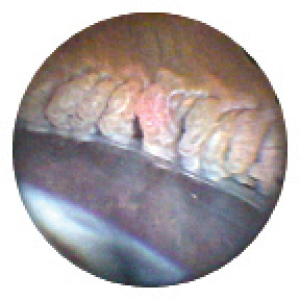
Figure 1. During ECP, the surgeon ablated the ciliary processes so as to shrink and whiten but not pop them.
.jpg)
Figure 2. In a retrospective case series of 24 patients who underwent combined laser cataract surgery and ECP, on average, IOP decreased by 5.7 mm Hg (29.1%; P = .03), and the mean reduction in medications was 1.1 (P < .001) at 1 year. Abbreviations: Pre-op, preoperative; POD, postoperative day; POW, postoperative week; POM, postoperative month; POY, postoperative year.
Because a primary objective of laser cataract surgery is addressing astigmatism, my colleague and I found it useful to investigate specifically the impact of ECP on the astigmatic outcomes of cataract surgery. We recently conducted a retrospective case series of 24 patients who underwent laser cataract surgery and ECP so as to evaluate the safety and efficacy of the combined treatment in patients with both cataract and glaucoma. The study included consecutive patients with a visually significant cataract, mild to moderate glaucoma for which they were using at least one drop, and at least 1 year of follow-up.4
Surgical technique was the same for all patients. I used the femtosecond laser to perform the capsulorhexis and lens fragmentation, followed by creation of the temporal corneal incision and paracentesis. I also used the laser to make arcuate incisions to minimize preoperative astigmatism. Thereafter, I removed the nucleus and cortex with minimal phacoemulsification, performed irrigation and aspiration, and implanted the IOL. Viscoelastic was then injected posterior to the iris to inflate the sulcus in preparation for ECP. I inserted the endoscope through the corneal incision(s), through the pupil, and into the sulcus in order to visualize the ciliary processes. Next, I treated all visible ciliary processes and the intervening crypts. The laser energy and distance from the ciliary processes were titrated based on individual response, but the level was usually between 200 and 300 mW. The laser treatment was enough to cause the ciliary processes to shrink and whiten but not to pop (Figure 1). Up to 270º can be treated through a single incision, and the entire ciliary ring can be treated through two corneal incisions. Once treatment was complete, I completely removed viscoelastic from the eye, administered an intracameral injection of triamcinolone acetonide (Kenalog; Bristol-Myers Squibb) into the anterior chamber, and rinsed out the corticosteroid, leaving a residual amount adherent to the iris. Performing ECP at the end of cataract surgery adds only a few minutes to procedural time.
One year after surgery, the mean IOP decreased 29.1% from 20.58 ±5.25 mm Hg at baseline to 14.88 ±2.03 mm Hg. In addition, the mean number of medications decreased from 1.58 ±0.78 to 0.46 ±0.59 (Figure 2). The majority of the patients did quite well visually, with a postoperative UCVA of 0.14 ±0.18 LogMAR and only a slight myopic shift, with a difference of 0.19 D from predicted outcomes. As expected, patients required a more prolonged regimen of anti-inflammatory medications that averaged 5.20 ±3.18 weeks.
Based on this study, ECP is neutral in its effect on refractive error, and it lowers IOP well. Although inflammation required more aggressive management with a prophylactic steroid in the anterior chamber, my colleague and I observed no increase in cystoid macular edema or persistent inflammation beyond the normal postoperative period.
FINANCIALS
ECP is reimbursed using code 66711, and the national average reimbursement is $650 for the physician. When combined with cataract surgery, the cataract surgery fee is reduced by 50%. The allowed amount for an ambulatory surgery center is $960, and the hospital reimbursement is $1,750. The cost for a semi-reusable triple-function laser endoscope from Beaver-Visitec Endo Optiks averages $100 to $133 per procedure. This does not include the capital cost of the equipment.
CONCLUSION
Overall, I find ECP to be an acceptable means of managing glaucoma in conjunction with cataract surgery. In addition to being very effective, the procedure is not exclusionary; I can combine it with an iStent Trabecular Micro-Bypass Stent (Glaukos), Trabectome (NeoMedix,), Trab360 or Visco360 (both from Sight Sciences), or any other procedure or instruments I feel would benefit the patient. ECP can also be performed before, after, or with any other glaucoma outflow procedure.
1. El-Saied HM, Foad PH, Eldaly MA, Abdelhakim MA. Surgically induced astigmatism following glaucoma surgery in Egyptian patients. J Glaucoma. 2014;23:190-193.
2. Saboori M, Kim J, Bhorade A, et al. The effect of endoscopic cyclophotocoagulation on refractive outcomes when combined with cataract surgery. Invest Ophthalmol Vis Sci. 2014;55:e-abstract 2533.
3. Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg. 2014;40:1313-1321.
4. Lee D, Noecker RJ. Combined femtosecond laser-assisted cataract surgery and endocyclophotocoagulation in treatment of patients with both cataract and glaucoma. Paper presented at: ASCRS/ASOA Symposium and Congress; April 18, 2015; San Diego, CA.
Robert J. Noecker, MD, MBA
• in practice with the Ophthalmic Consultants of Connecticut in Fairfield, Connecticut
• assistant clinical professor, Yale University School of Medicine, New Haven, Connecticut
• clinical professor of surgery, Netter School of Medicine, Quinnipiac University, North Haven, Connecticut
• noeckerrj@gmail.com
• financial disclosure: consultant to Beaver-Visitec Endo Optiks


