Why has there been so much buzz about the Light Adjustable Lens (LAL; Calhoun Vision) for so long when this unique IOL is only now nearing FDA approval in the United States? One reason is that interest in the accuracy and superior outcomes that refractive cataract surgery can provide is at an all-time high. Unlike any other IOL, the LAL is designed to empower surgeons to achieve predictable, high-quality outcomes by allowing noninvasive adjustment after implantation. The implant has received the CE Mark and is approved for use in Europe, where it has been commercially available since 2008. Overseas, surgeons and patients have seen inspiring results, but here in the United States, we are still waiting.
The LAL is making good progress toward FDA approval. Enrollment in a randomized, multicenter, 600-eye phase 3 clinical study is more than 80% finished and should be completed early this year. Game-changing technology takes more time to come to market than most people realize—typically, an average of 17 years passes before novel research ideas reach clinical practice.1
BACKGROUND AND CONCEPT
The LAL’s concept was born in the late 1990s. Because it represents a major breakthrough in lens implant surgery, it is subject to greater scrutiny than a “me-too” device or an improvement to an already existing product.
What makes the lens such a paradigm shift is that it is the world’s first IOL that can be modified in one or two visits after it is implanted, with the goal of tailoring refractive power to a patient’s specific, individual needs. The lens is made up of proprietary biocompatible, photosensitive materials called macromers. When illuminated with ultraviolet light of a certain profile, the macromers are photopolymerized, leading to a change in the shape—and therefore the power—of the lens. Once the LAL has been adjusted and the patient is happy with his or her visual results, the surgeon performs a “lock-in” procedure to prevent any additional power change.
EUROPEAN RESULTS WITH THE LAL
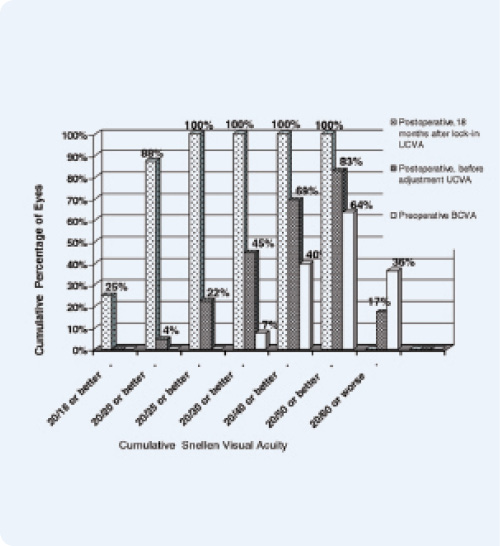
Figure 1. Postadjustment UCVA compared with preadjustment UCVA (121 eyes, 18 months after lock-in).
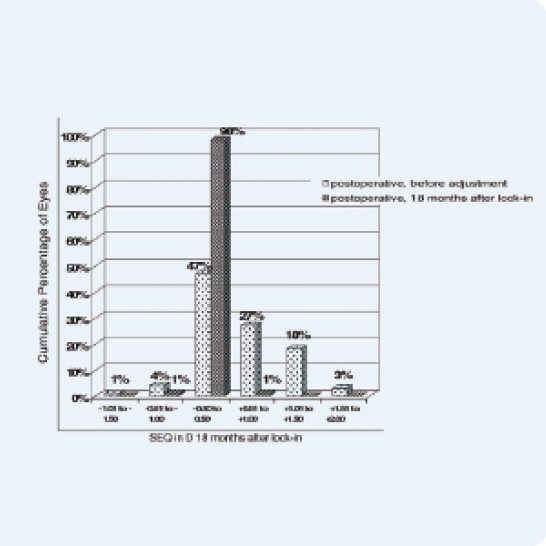
Figure 2. Accuracy of spherical equivalent refraction (121 eyes, 18 months after lock-in).
Although the fact that the LAL has a CE Mark and has been sold for years in Europe does not influence or expedite the device’s FDA approval, it does provide US surgeons with a sneak peek into what we can expect from the lens once approved. In fact, in one study in Germany in which 121 eyes were implanted with the LAL and subsequently adjusted, 88% of patients had a visual acuity of 20/20 or better, and 100% saw 20/25 or better 18 months after lock-in. Those results also showed that 98% of patients were within 0.50 D of the targeted manifest refraction spherical equivalent (Figures 1 and 2).2
As investigators in the US phase 3 study, my colleagues and I have been very impressed with the LAL’s performance to date. It allows us a level of precision and predictability that we simply have not seen with existing IOL technology. Our early results, combined with the exceptional data from Europe, lead me to believe that the IOL has the potential to transform lens implant surgery.
CONCLUSION
With nearly 22 million Americans aged 40 years and older affected by cataracts, the US market is primed for the LAL. Momentum and a convergence of factors are pushing us in the direction of the LAL, but we must be patient. Calhoun Vision is taking the right approach by thoroughly testing the LAL in a randomized, multicenter trial and working with the FDA to ensure the lens’ safety and effectiveness for patients.n
1. Westfall J, Mold J, Fagnan L. Practice-based research–“Blue Highways” on the NIH roadmap. JAMA. 2007;297:403-406.
2. Hengerer FH, Dick HB, Conrad-Hengerer I. Clinical evaluation of an ultraviolet light adjustable intraocular lens implanted after cataract removal: eighteen months follow-up. Ophthalmology. 2011;118(12):2382-2388.
Damien Goldberg, MD
• private practice at Wolstan & Goldberg Eye Associated, Torrance, California
• (310) 543-2611; goldbed@hotmail.com; Twitter @damiengoldberg1
• clinical investigator for Calhoun Vision


