The Implantable Miniature Telescope (IMT; VisionCare Ophthalmic Technologies), FDA approved in 2010, is now available to patients aged 65 years and older living with bilateral, end-stage, age-related macular degeneration (AMD). These patients have either geographic atrophy or disciform scarring involving the fovea in both eyes, and their AMD is deemed uncorrectable by any other treatment, including glasses, vitamins, drugs, or cataract surgery. In brief, the device’s technology is based on wide-angle micro-optics that, in combination with the optics of the cornea, create a telephoto system that magnifies objects in view and thereby minimizes the central scotoma. The IMT is proven to restore vision and improve patients’ quality of life.1
IMPROVED ACCESS
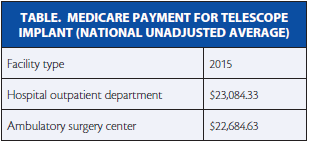
The implant is the only surgical treatment for end-stage AMD. Since 2011, it has been a Medicare-eligible procedure performed in a hospital setting, because reimbursement was set up via the Outpatient Prospective Payment System. However, in the Centers for Medicare & Medicaid Services’ CY 2015 Hospital Outpatient Prospective Payment System and Ambulatory Surgery Center (ASC) Payment System final rule, the agency announced it will reimburse the IMT under a revised Ambulatory Payment Classification or APC designation, APC 0351, Level V Intraocular Procedures, and at levels comparable to the 2015 hospital setting, which will have meaningful implications for the cornea surgeon participating in the CentraSight treatment program (Table).
CentraSight is VisionCare Ophthalmic Technologies’ comprehensive treatment program, which helps patients follow the steps necessary for proper diagnosis, surgical evaluation, implantation, and postoperative care. The cornea surgeon is one of several health care professionals evaluating and working directly with the telescope candidate. By moving the surgery into the ASC, providers will appreciate being able to more conveniently offer the telescope procedure in the setting where they already provide up to 80% of ophthalmic procedures. The more important outcome is that patients will enjoy greater access to a procedure that has not only been shown to significantly improve quality of life but is also cost-effective by conventional standards and compared to other medical therapies.2
The IMT is the only surgical treatment for end-stage AMD.
PRECISION AND PLACEMENT
Placing the device can be challenging for surgeons, because the IMT cannot be treated simply as a large lens. Rather, the unique configuration and material composition of the IMT require surgeons to be mindful of the relationship between the device and the anterior chamber throughout the procedure. Specifically, the incision must be adequately sized at the limbus to accommodate the stiff haptics of the carrier plate with minimal intraoperative manipulation. Liberal use of ophthalmic viscoelastic devices is needed to facilitate the placement so as to minimize endothelial cell loss.3 The American Medical Association Current Procedural Terminology Panel granted this procedure (which is a hybrid cornea-cataract procedure) a unique code: 0308T “Insertion of ocular telescope prosthesis including removal of crystalline lens.”
After the device’s implantation, images seen through the straight-ahead vision are rendered approximately 2.7 times larger than the natural lens provides. The magnification allows central images to be seen by viable perimacular retina around the areas of central scotoma. (Worth noting, studies indicate that cataract removal in the end-stage AMD patient does not provide clinically meaningful visual benefit, as does the IMT.4) Postoperatively, the surgeon prescribes a standard regimen of eye drops in addition to atropine dilating drops for 1 month.
Antivascular endothelial growth factor therapies are the standard of care for recurrent choroidal neovascularization. A recent publication has shown that optical coherence tomography can be used through the implant to monitor the fundus and that injecting antivascular endothelial growth factor agents behind the implant is very similar to injecting them in a phakic eye.5
Other Implantable Magnification Technology
Several devices have been proposed to restore the visual potential of patients with age-related macular degeneration (AMD). The concept was first introduced in the form of implantable telescopes in the mid-1990s,1,2 but applicability remained limited due to inherent compromises in visual function. In 2007, Orzalesi and coauthors described the first applications of implanted IOLs designed to magnify images.3 Although it results in fewer visual compromises, the Intraocular Lens for Visually Impaired People (IOL VIP, distributed in the United Kingdom by Veni Vidi) does necessitate an 8-mm incision to implant the dual, nonfoldable lenses. In a more recent iteration of the technology called the IOL VIP Revolution, both implants are placed in the capsular bag and separated by a silicone ring. However, the surgery requires an 8-mm incision and capsulorhexis, and it may be technically challenging to align the dual lenses while manipulating the silicone ring.
Bobby Qureshi, BSc, MBBS, FRCSOphth, a consultant ophthalmic surgeon and medical director at London Eye Hospital, has invented a device that he believes solves many of these issues associated with implants for patients with AMD. The iolAMD is a dual lens system, with one lens implanted in the sulcus and the other in the capsular bag to create a Galilean effect. The plate-haptic design is inserted through a 3-mm incision and requires a 5-mm capsulorhexis. The current model offers approximately 1.3× magnification with about 3° of prism to offset the image away from areas of pathology. Prior to surgery, patients are selected via a lens simulator designed to test feasibility. “We have something that can be performed by any cataract surgeon, adding about a minute to a standard cataract surgery,” Dr. Qureshi said in an interview with Cataract & Refractive Surgery Today’s sister publication, Retina Today.
The device has so far been implanted in almost 100 patients with early and advanced AMD, with close to 4 months of follow-up on 18 eyes of 12 patients. Overall, 67% of patients had gains in mean distance BCVA, and 50% improved in near BCVA; side effects have been minimal (ie, no significant change in IOP and only a small, insigificant reduction in endothelial cell counts).4 According to Dr. Qureshi, important criteria for patient selection are phakic status with a minimal cataract and a refraction of ±4.00 D with less than 3.00 D of astigmatism. Patients who should be excluded include those who are pseudophakic or aphakic or who have advanced glaucoma, zonular instability, or pigment dispersion.
1. Mayer A. Clinical experience with the Ben-Sira teledioptric system for use in age-related macular degeneration. Ger J Ophthalmol. 1996;5:229-232.
2. Lipshitz I, Lowenstein A, Reingetwirtz M, Lazar M. An intraocular telescopic lens for macular degeneration. Ophthalmic Surg Lasers. 1997;28:513-517.
3. Orzalesi N, Pierrottet CO, Zenoni S, Savaresi C. The IOL-VIP System: a double intraocular lens implant for visual rehabilitation of patients with macular disease. Ophthalmology. 2007;114(5):860-865.
4. Hengerer F. Personal experience and clinical results. iolAMD: a new surgical approach to managing dry AMD. Presented at: XXXII Congress of the ESCRS; September 14, 2014; London, UK.
CONCLUSION
The CentraSight program helps optimize caring for the IMT patient by leveraging the talents of a team of specialists. The cornea-trained cataract surgeon has a responsibility not only to understand the patient’s visual and functional goals but also to work closely with retina and low vision specialists when making treatment evaluations. It is also true that many long-term AMD patients will not be aware that the implant is a possibility. Thus, all providers in the eye care community can serve as a first link to CentraSight.n
1. Hudson HL, Lane SS, Heier JS, et al. Implantable miniature telescope for the treatment of visual acuity loss due to end-stage age-related macular degeneration: one-year results. Ophthalmology. 2006;113:1987-2001.
2. Brown GC, Brown MM, Lieske HB, et al. Comparative effectiveness and cost-effectiveness of the Implantable Miniature Telescope. Ophthalmology. 2011;118:1834-1843.
3. Colby KA, Chang DF, Stulting RD, Lane SS. Surgical placement of an optical prosthetic device for end-stage macular degeneration: the Implantable Miniature Telescope. Arch Ophthalmol. 2007;125:1118-1121.
4. Farah SE. The impact of cataract surgery on preexisting retinal disease. EyeNet Magazine. September 2010.
www.aao.org/publications/eyenet/201009/pearls.cfm. Accessed November 3, 2014.
5. Joondeph, BC. Antivascular endothelial growth factor injection technique for recurrent exudative macular degeneration in a telescope implanted eye. Retin Cases Brief Rep. 2014;8(4):342-344.
Gerard D’Aversa, MD
• partner in Ophthalmic Consultants of Long Island in New York
• (516) 330-6935; gdaversa@ocli.net.
• acknowledged no relevant financial interest
Glenn Stoller, MD
• specialist in medical and surgical diseases of the retina, vitreous and macula at Ophthalmic Consultants of Long Island in New York
• (516) 766-2519;gstoller@ocli.net.
• acknowledged no relevant financial interest


