Corneal allogenic addition technology incorporates allogenic tissue or volume into the cornea to achieve various outcomes. In 2015, I introduced a technology called corneal allogenic intrastromal ring segments, better known as CAIRS.1 It refers to any allogenic tissue implanted intrastromally in the form of a ring or segment. Currently, the most common source of allogenic tissue for CAIRS is donor corneas. Other sources, however, such as processed CAIRS or alternative allogenic tissue, may be used in future applications of the technology.
Background on Corneal Allogenic Addition Technology
The field of addition technology was pioneered by José Ignacio Barraquer, MD, FACS, FRCOphth, the father of refractive surgery. He also established the Barraquer thickness law, which holds that adding tissue to the periphery of the cornea flattens it. In his work, Barraquer used both synthetic plexiglass and allogenic lenticule addition.2 Synthetic inlays led to melting and necrosis, but allogenic inlays performed better.
Allogenic addition technology offers advantages over synthetic addition technology, primarily in terms of biocompatibility and a reduced risk of complications.3-5 A greater volume of allogenic addition technology may be implanted more superficially and in a smaller optical zone compared to synthetic segments, resulting in a more significant refractive effect. An allogenic approach also lowers the risk of complications such as extrusion, intrusion, migration, necrosis, and neovascularization.
Development of CAIRS
For many years, I’ve dedicated my professional career to the treatment of patients with keratoconus. I am passionate about improving their quality of life. This complex disease often affects young individuals, and, unfortunately for patients with advanced keratoconus, their corneas often become too thin for the standard CXL treatment. In 2012, driven by the need for an alternative, I pioneered a contact lens–assisted CXL technique designed to halt disease progression in such cases.1,6 Much like other CXL techniques, this approach helped stabilize the disease but did little to enhance patients’ visual acuity because of the minimal impact on topography and the steepness of the corneal cone. Thus, for these patients, deep anterior lamellar keratoplasty (DALK) frequently emerged as the next viable option. DALK, however, is not without its own set of challenges, including a range of intra- and postoperative complications such as the risk of Descemet membrane perforation during surgery, Urrets-Zavalia syndrome, irregular astigmatism, and various suture-related complications. Around this time, the alternative of synthetic intrastromal corneal ring segments (ICRSs) was also considered, but these, too, were associated with numerous risks and had a limited effect, rendering them unsuitable for patients with advanced keratoconus.
Consequently, I found myself wondering whether the effect of ICRSs could be achieved more safely using allogenic tissue. I also hypothesized that a more superficial implantation might have a greater impact. This line of thinking marked the genesis of the CAIRS technique in 2015.
Other allogenic addition technologies exist, but most involve lenticular inlays that cover the visual axis. Because every allogenic inlay carries a small risk of haze and rejection, complications affecting an inlay that covers the visual axis could conceivably have an impact on the central cornea and thereby on vision. This could result in a variety of complications ranging from loss of contrast sensitivity, glare, and scatter to a decrease in visual acuity.
In contrast, the CAIRS technique leaves the central visual axis untouched by the surgeon, thus reducing the risk of complications that could compromise the patient’s vision.1 Complications with CAIRS, if any, are thus limited to the midperipheral, nonvisual part of the cornea.
Mechanisms of CAIRS
CAIRS involves the insertion of customized allogenic tissue segments into circular channels within the corneal midstroma. CAIRS technology functions similarly to but more effectively than synthetic ICRSs (Figure 1). This superiority stems from the greater volume of tissue that can be implanted within a smaller optical zone and at a more superficial plane than synthetic segments permit.
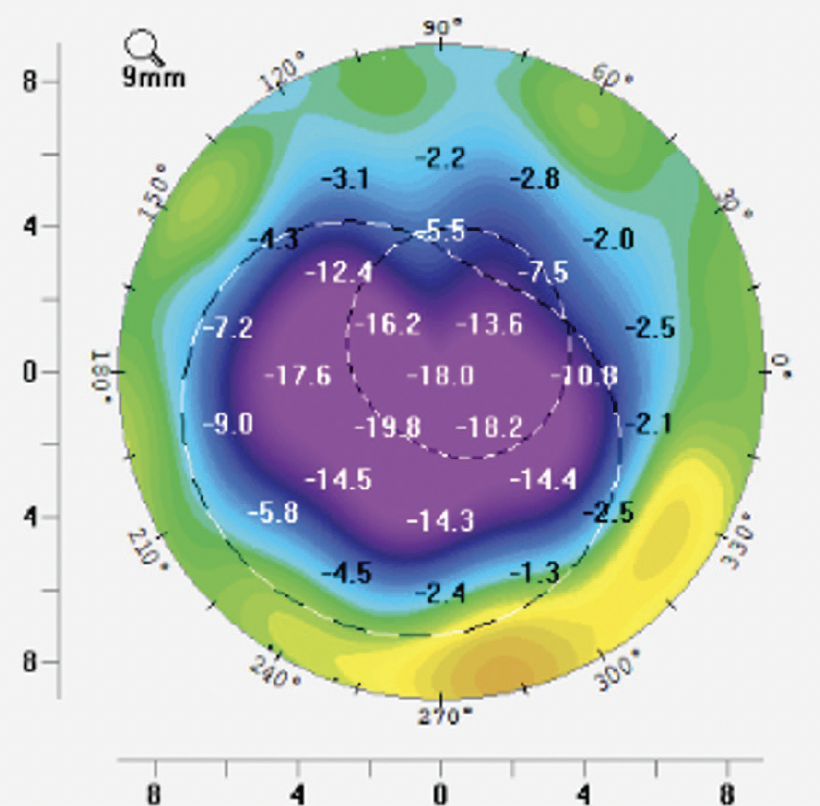
Figure 1. Difference map of front axial curvature showing a large amount of flattening after CAIRS implantation.
CAIRS can enhance both the quality and quantity of patients’ vision through four mechanisms.1 First, the procedure increases the volume in the affected midperipheral cornea, leading to the flattening and regularization of the central cornea. Second, CAIRS promotes epithelial remodeling. Third, it induces an arc-shortening effect. Fourth, it thickens the area of implantation and helps redistribute corneal stress forces, potentially playing a role in stabilizing the cornea.
Advantages
Ultrasmall tissue strip. CAIRS does not necessitate the removal of any part of the cornea as in transplantation or topography-guided PRK. Such a procedure, if performed, could further destabilize a thin, keratoconic cornea. Notably, CAIRS is an additive technique as opposed to subtractive or replacement therapies.
Small tissue volume. The volume of tissue implanted during the CAIRS procedure is significantly smaller than that used in traditional transplantation methods. Furthermore, it does not necessitate the implantation of the more antigenic epithelium or endothelium. These factors collectively reduce the potential risk of rejection. Importantly, as mentioned previously, tissue is implanted only in the midperiphery, ensuring that vision remains unaffected, even in the worst-case scenario of rejection.
Simplicity. Compared to standard corneal transplantation, the CAIRS procedure is simpler and avoids potential intraoperative complications associated with keratoplasty. These include complications related to DALK such as micro- and macroperforations, suture-related irregular astigmatism, and Urrets-Zavalia syndrome.
No intraocular steps. The procedure is therefore safer compared to corneal transplantation.
Short recovery time. Visual rehabilitation is faster after CAIRS versus corneal transplantation because the former is a sutureless procedure.
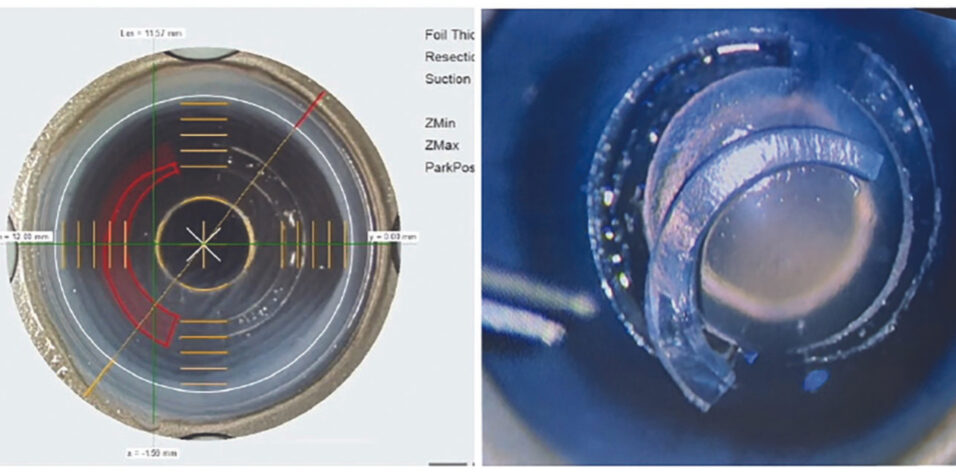
Customizability. The technology accommodates customization to each patient’s needs, encompassing variations in segment shape, thickness, arc length, and depth of implantation (Figure 2). Excellent precision in these adjustments can be achieved using tools such as the Jacob CAIRS customizer (Epsilon Instruments) and the Jacob double-bladed trephine (Madhu Instruments). Alternatively, femtosecond laser technology can be used. Shady T. Awwad, MD, and I have partnered with Ziemer Ophthalmic Systems to develop CAIRS software for the company’s Femto LDV Z8 laser. The laser creates tapered segments and circular intrastromal channels for CAIRS insertion. Following the femtosecond laser creation of CAIRS, the customized segment is implanted into circular channels of the desired arc length, also created in the patient’s cornea with the LDV Z8 laser (Figure 3).

Figure 2. A customized, broad-edged, tapered CAIRS is implanted to reduce coma. The left and right images were obtained pre- and postoperatively, respectively. The central image in the top row displays the difference map for axial curvature. Vertical coma, sphere, and spherical equivalent decrease significantly. The cone becomes more centralized and symmetric over the pupil, reducing the distortion of vision and improving the quality and quantity of vision. The tapering pink arrow shows the direction of the desired gradient effect obtained with customized CAIRS.

Figure 3. CAIRS treatment is customized with the Femto LDV Z8 (A). The segment is lifted from the donor cornea (B).
Conclusion
CAIRS represents a significant step forward in the management of patients with keratoconus. The procedure can be combined with CXL to tackle progressive disease. The combined approach has the potential to improve corneal topography and visual acuity and preclude the need for corneal transplantation in many individuals with thin corneas. Moreover, CAIRS can be used in eyes with mild keratoconus to improve both corneal topography and quality of vision.
CAIRS has been used successfully in patients with post-LASIK ectasia. Beyond its applications for keratoconus and post-LASIK ectasia, CAIRS may also be beneficial for the treatment of other corneal ectatic disorders such as pellucid marginal degeneration. Additionally, it holds potential for reducing astigmatism in eyes with thin corneas.7
1. Jacob S, Patel SR, Agarwal A, Ramalingam A, Saijimol AI, Raj JM. Corneal allogenic intrastromal ring segments (CAIRS) combined with corneal cross-linking for keratoconus. J Refract Surg. 2018;34(5):296-303.
2. Barraquer JI. Queratoplatica refractiva. Estudios e informaciones Oftalmologicas. 1949;2:10.
3. Kozhaya K, Mehanna CJ, Jacob S, Saad A, Jabbur NS, Awwad ST. Management of anterior stromal necrosis after polymethylmethacrylate ICRS: explantation versus exchange with corneal allogenic intrastromal ring segments. J Refract Surg. 2022;38(4):256-263.
4. Carpel EF, Santilli C, Maltry A. Long-term viability of allogenic donor stroma. Indian J Ophthalmol. 2020;68(12):3057-3059.
5. Abad JC, Gomez IC, Henriquez MA, Donado JH. Biomicroscopic findings and management of anterior stromal necrosis after long-term implantation of Intacs. Am J Ophthalmol. 2020;220:170-176.
6. Srivatsa S, Jacob S, Agarwal A. Contact lens assisted corneal cross linking in thin ectatic corneas – a review. Indian J Ophthalmol. 2020;68(12):2773-2778.
7. Kalas T, Gunn D. Corneal allogenic intrastromal ring segment implantation for post LASIK ectasia. Journal of Refractive Surgery Case Reports. 2023;3(1):e1–e4