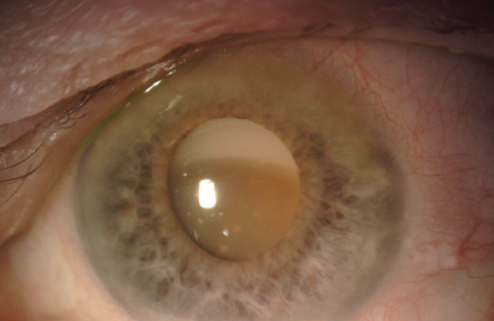
An 84-year-old Russian woman presents for a cataract surgery evaluation. The patient has a history of a failed graft, retinal detachment, and glaucoma in her right eye. Visual acuity is light perception in her right eye and counting fingers in her left. A slit-lamp examination of the patient’s left eye finds a dense cataract, an inferiorly dislocated lens, loose zonules, and phacodonesis (Figure). The cornea is clear, and endothelial cell density is normal. B-scan ultrasonography shows no retinal detachment. The IOP measures 15 mm Hg. Examination reveals no other significant findings.
How would you proceed? What form of anesthesia would you use? What would your preferences be in terms of surgical technique and IOL?
—Case prepared by Audrey R. Talley Rostov, MD

ASHVIN AGARWAL, MS
Because the patient is almost blind and her right eye has undergone multiple surgeries, I would first attempt to restore some vision in her left eye. It has a diseased bag-lens complex and a progressive condition that threatens the longevity of the bag-IOL complex. My procedures of choice would be manual small-incision cataract surgery (SICS) to explant the cataractous lens, followed by intrascleral haptic fixation of an IOL (glued IOL technique) under peribulbar anesthesia.
I would begin by making a conjunctival peritomy at the 3 and 9 clock hours and two partial-thickness scleral flaps located 180º apart. I would also create the SICS tunnel but leave it unopened. After performing continuous curvilinear capsulorhexis, in order to ensure that the zonules were manipulated as little as possible, I would pull the lens into the anterior chamber. Next, I would open the SICS wound and extract the lens using a viscoexpulsion technique, which, in my experience, minimizes stress on the zonules. Examination with iris hooks might then be warranted.
I would use the space under each scleral flap to make a sclerotomy, which I would then use to perform anterior vitrectomy and externalize the haptics. After ensuring that the haptics were well tucked into the Scharioth tunnels, I would seal them with glue. I would end the case with pupilloplasty to optimize the outcome.

MARJAN FARID, MD
A controlled plan of action would be extremely important in this case. The first steps would be to set realistic expectations for the patient and to select the correct approach to anesthesia. I would explain to the patient the severity of her situation and make sure she understood that a second surgery with a team of retina doctors will be necessary if the cataract cannot be safely removed via an anterior approach. Having retina colleagues available on standby would be a good idea.
The degree of zonular laxity and loss will ultimately determine the overall success of this case through an anterior approach. I would use a femtosecond laser to create a capsulotomy centered on the lens and to perform nuclear fragmentation. I would employ capsular hooks to help center the lens-capsule complex and maintain its centration and stability. I would perform nuclear disassembly with a slow and deliberate chopping technique, being careful to minimize stress on the remaining zonules. A capsular tension ring might help to stabilize localized areas of zonular weakness, but I prefer to insert the device as late as possible to minimize trapping of cortical material around the capsular equator, which can then be difficult to remove with irrigation and aspiration.
If I was able to safely remove the nucleus in its entirety through an anterior approach, I would then use an Ahmed Capsular Tension Segment (CTS, Morcher, US distributor FCI Ophthalmics) with scleral fixation to stabilize the intact capsule. In some cases, two or even three CTSs may be needed to create 360º stability of the capsule complex. My preference would be a three-piece IOL because its spring-like haptics would provide some tension within the capsule. A three-piece IOL would also allow scleral or iris fixation if needed.

P. DEE G. STEPHENSON, MD, FACS
I would have more than one surgical plan ready. No matter the technique, I would expect its execution to require longer than usual, and I would be prepared for supplemental anesthesia to be necessary.
The patient is at increased risk of complications, additional surgery may be needed, and her recovery time and use of medication are likely to be longer than usual. It will be important to explain all of these things to the patient before proceeding.
The maturity of the lens will require a larger capsulotomy (5.5–6 mm) than I typically create. The Zepto Capsulotomy System (Mynosys Cellular Devices) could be useful in this case. My preference, however, would be to use a femtosecond laser (Lensar Laser System, Lensar) for the capsulotomy because it can be customized to the density of the cataract. I would check that the capsule was free-floating; if I was unsure, I would stain it with trypan blue dye.
I would perform gentle hydrodissection. Because this type of cataract tends to be fibrous or leathery at the posterior portion of the lens, the miLoop (IanTech) might help to cleave the lens into pieces. Care must be taken not to press down with this device and thus damage the zonules. I would protect the endothelium with a dispersive OVD and monitor the zonules as cataract surgery progressed. I would have at the ready iris hooks, capsular tension rings, CTSs, and pupil expanders. I would also have more than one type of IOL available in anticipation of various possible circumstances (eg, sulcus fixation with optic capture, scleral fixation of the IOL, or use of an anterior chamber IOL).
If all had gone well to this point, I would exercise caution during cortical removal so as to protect the posterior capsule. If the bag and zonules were stable, I would place the IOL in the bag or perform reverse optic capture. If the nucleus precipitated posteriorly, I would insert an appropriately sized and powered anterior chamber IOL and leave the rest of the case for a retina specialist.

WHAT I DID: AUDREY R. TALLEY ROSTOV, MD
Owing to some medical issues, the patient waited 4 months before scheduling cataract surgery. At that point, the lens nucleus had dropped entirely into the vitreous. Based on the medical complexity of the case (eg, cardiac issues and concerns about general anesthesia), the retina specialist decided to observe cataract surgery rather than schedule a joint procedure. I selected an anterior chamber IOL, which was also the preference of the retina surgeon. My choice of IOL reflects my concern that potential future retinal surgery could disturb the implant.
Cataract surgery was uneventful, and the patient had 20/40 UCVA postoperatively.




