Microinvasive glaucoma surgery (MIGS) is an expanding field. Novel MIGS devices aim to reduce IOP and decrease patients’ dependence on glaucoma medications while avoiding the risks of traditional glaucoma surgery such as trabeculectomy and tube shunts. Today, most of the popular MIGS procedures involve stent devices that are designed to enhance aqueous outflow via Schlemm canal or to create new outflow pathways into the suprachoroidal or subconjunctival space. In this article, we summarize evidence and data regarding the clinical efficacy of MIGS stent devices currently available in the United States.
iStent and iStent inject
The iStent (Glaukos) is a trabecular microbypass shunt intended to lower IOP by direct cannulation of Schlemm canal (Figure 1). Large, prospective, randomized controlled trials have evaluated the device’s safety and efficacy when implanted in combination with phacoemulsification in patients with open-angle glaucoma (OAG).1,2 In these trials, more eyes that received the iStent met the primary efficacy outcome of unmedicated IOP of 21 mm Hg or less at both 1 and 2 years after surgery (72% in the iStent group vs 50% in the phaco-only group at 1 year [P < .001]; 61% vs 50% at 2 years [P = .036] in the same two groups, respectively). Additionally, more iStent eyes achieved a reduction in unmedicated IOP of 20% or more at 1 year after surgery (66% in the iStent group vs 48% in the phaco-only group [P = .003]), although the effect was not statistically significant at 2 years (53% vs 44% [P = .09]). Similarly, postoperative medication use was significantly lower in iStent eyes at 1 year (0.2 medications in the iStent group vs 0.4 in the phaco-only group [P = .011]), but not at 2 years (0.3 vs 0.5 [P > 0.05]).

Figure 1. The first-generation iStent (left) has a self-trephining tip that is passed through the trabecular meshwork and advanced parallel to Schlemm canal until only the snorkel is visible from the anterior chamber. The pointed end of the iStent inject, the second-generation iStent device (right), is deployed perpendicularly through the trabecular meshwork into Schlemm canal.
These studies demonstrated the safety and modest efficacy of the iStent relative to cataract surgery alone; however, subsequent studies have shown greater effect lasting over longer periods. In an uncontrolled case series, Arriola-Villalobos et al followed 13 patients with mild to moderate OAG who received an iStent in combination with phaco for 5 years.3 At that follow-up interval, mean IOP reduction relative to baseline was 16.3%, medication use had decreased by 63.6%, and the unmedicated IOP was 21 mm Hg or less in 62% of patients. In a small trial, Fea et al randomly assigned 36 patients with OAG to receive either phacoemulsification alone or with an iStent.4 Twenty-four patients completed 4 years of followup, at which time they underwent a 1-month washout of glaucoma medications. Although IOP was similar in both study groups before washout (15.9 mm Hg in the iStent group vs 17 mm Hg in the phaco-only group [P > .05]), IOP after washout was significantly lower in the iStent group (17.5 mm Hg vs 20.4 mm Hg [P = .02]), leading the authors to conclude that the iStent maintains long-term efficacy in lowering IOP.
Other studies have evaluated the implantation of multiple iStents without concurrent cataract surgery. Katz et al randomly assigned 119 patients with mild to moderate OAG whose IOP was not well controlled on two medications to receive one, two, or three iStents.5 Twelve months after surgery, more than 85% of patients in all groups had both a reduction in unmedicated IOP of 20% or more and an unmedicated IOP of 18 mm Hg or less. More multiple-stent patients had unmedicated IOP of 15 mm Hg or less, although this difference was not statistically significant (64.9% in the one-stent group, 85.4% in the two-stent group, and 92.1% in the three-stent group).
To evaluate the use of multiple iStents as a first-line treatment method, Vold et al randomly assigned 101 patients with newly diagnosed OAG either to two iStents implanted without concurrent cataract surgery or to medical therapy with travoprost.6 After 3 years, 91% of iStent patients had unmedicated IOP of 18 mm Hg or less, and 62% had unmedicated IOP of 15 mm Hg or less; among the travoprost patients, these figures were 79% and 21%, respectively (statistical significance not indicated).
The iStent inject (Figure 1), a second generation iStent device, received FDA approval in June. Unlike the original iStent, the iStent inject is designed to be deployed perpendicularly into the trabecular meshwork to reduce the technical challenge of stent implantation. Additionally, each injector is loaded with two stents. To date, one multicenter randomized trial has been performed with the iStent inject, in which 192 eyes with OAG not well controlled on one medication were randomly assigned to receive either a fixed combination of latanoprost and timolol or two iStent inject devices as a solo procedure (ie, not in combination with cataract surgery).7 At 12-month followup, a similar proportion of patients in both treatment groups achieved unmedicated IOP reduction of 20% or more (94.7% of iStent inject eyes vs 91.8% of medication eyes [P > .05]), but a higher proportion of patients in the iStent inject group achieved an unmedicated IOP reduction of 50% or more (53.2% vs 35.7% [P = .02]).
Hydrus
Like the iStent, the Hydrus Microstent (Ivantis) is designed for ab interno cannulation of Schlemm canal (Figure 2). Both the Hydrus and the iStent have an inlet segment in the anterior chamber, but the Hydrus also incorporates a segment that dilates and scaffolds a quadrant of Schlemm canal. The recently published Hydrus Microstent for Lowering IOP in Glaucoma Patients Undergoing Cataract Surgery (HORIZON) study was a prospective, multicenter, single-masked randomized controlled trial that evaluated the safety and efficacy of the device used as an adjunct to cataract surgery.8 A total of 369 eyes with mild to moderate OAG on at least one medication were randomly assigned to receive a Hydrus stent immediately after phacoemulsification; 187 eyes underwent phacoemulsification only. In that study, the Hydrus significantly outperformed phacoemulsification alone in both the primary and secondary study endpoints, with the magnitude of effect in favor of Hydrus increasing between 12 and 24 months of followup. At 24 months, 77.3% of Hydrus eyes achieved 20% or greater reduction in unmedicated modified diurnal IOP (MDIOP), compared with 57.8% of phaco-only eyes (P < .001). The average reduction in MDIOP was 7.6 mm Hg in the Hydrus group, compared with 5.3 mm Hg in the phaco-only group (P < .001). Additionally, prior to medication washout at 24 months, 78% of Hydrus eyes were medication-free, compared with 48% in the phaco-only group (P < .001).

Figure 2. The portion of the Hydrus Microstent that resides in Schlemm canal is longer than the corresponding segment of the iStent, thereby dilating and scaffolding about 3 clock hours of Schlemm canal.
Xen Gel Stent
Unlike the iStent and Hydrus, which aim to enhance aqueous outflow via Schlemm canal (ie, the conventional pathway), the Xen Gel Stent (Allergan) is designed to create a new outflow pathway into the subconjunctival space—much like a trabeculectomy or traditional tube shunt procedure but via an ab interno approach (Figure 3). Also, like traditional filtering surgeries, the Xen is intended for the management of refractory OAG that is unresponsive to maximum tolerated medical therapy (the FDA-approved indication for the device).
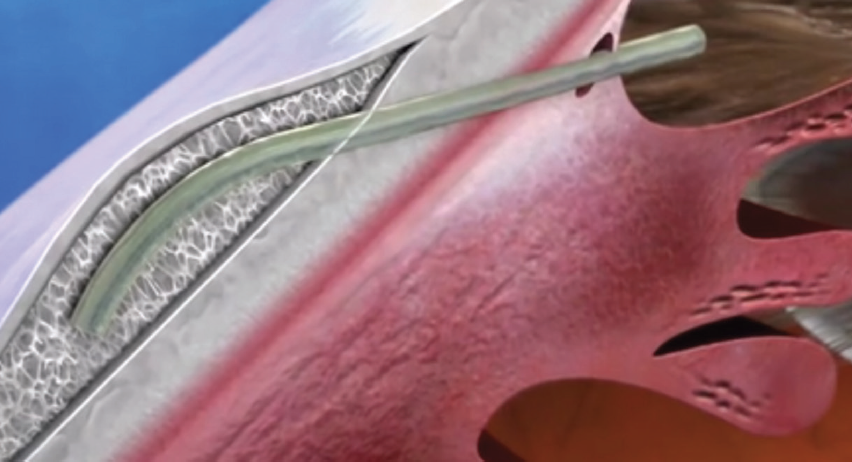
Figure 3. The 6-mm Xen Gel Stent is made of crosslinked gelatin that hydrates, softens, and becomes flexible after implantation. It is passed through the sclera via an ab interno approach to create a new aqueous outflow pathway into the subconjunctival space.
The largest multicenter study of the Xen to date retrospectively compared outcomes in 354 consecutive patients with refractory glaucoma who received either the Xen or trabeculectomy, both procedures performed with mitomycin C.9 Overall, the results showed similar rates of failure (defined as IOP above predefined thresholds or need for medications, reoperation, or loss of light-perception vision) for both the Xen and trabeculectomy. There was a significant interaction between preoperative BCVA and risk of failure, with the Xen outperforming trabeculectomy in eyes with BCVA better than 0.3 logMAR.
In a prospective, multicenter case series, Grover et al reported clinical results for 65 patients with refractory glaucoma (defined as IOP above goal on maximum tolerated medical therapy or failure of prior cilioablative or filtering procedure).10 Excluding four patients with missing 12-month followup data and nine patients requiring a secondary glaucoma surgery by that timepoint, 88.5% of patients had an IOP reduction of 20% or more on the same or fewer glaucoma medications, with MDIOP decreasing on average from 25.1 to 15.9 mm Hg, an average reduction of more than 9 mm Hg. Medication use decreased from an average of 3.5 agents at baseline to 1.7 at 12 months.
CyPass
The CyPass Micro-Stent (Alcon) represents a third category of MIGS stent; it was designed to augment uveoscleral outflow by creating an aqueous outflow pathway into the suprachoroidal space (Figure 4). In August, Alcon voluntarily withdrew the CyPass from the global market after 5-year outcomes data from the Study to Assess Long-Term Safety of the CyPass Micro-Stent in Patients Completing the COMPASS Trial (COMPASS-XT) demonstrated greater endothelial cell loss in eyes receiving Cypass compared with controls. Announcing the withdrawal, company officials said Alcon intends to partner with the FDA and other regulators to explore labeling changes that would support the reintroduction of the CyPass in the future.

Figure 4. The CyPass Micro-Stent is a flexible, fenestrated polyimide stent inserted through the angle at the level of the scleral spur into the supraciliary space. Alcon recently voluntarily withdrew the device from the global market.
The CyPass is a flexible, fenestrated polyimide stent that is inserted through the angle at the level of the scleral spur into the supraciliary space. The initial COMPASS trial was a 2-year, prospective, multicenter randomized controlled trial that evaluated the safety and efficacy of CyPass as an adjunct to cataract surgery.11 After uncomplicated phacoemulsification, 374 eyes with OAG on zero to three medications received the CyPass; 131 eyes underwent phacoemulsification only. At 2 years, 73% of CyPass eyes achieved 20% or greater reduction in unmedicated MDIOP, compared with 58% of phaco-only eyes (P = .002). Reduction in average MDIOP was 7.4 mm Hg in the CyPass group compared with 5.4 mm Hg in the phaco-only group (P < .001). Additionally, phaco-only eyes required three times more medications than CyPass eyes (0.6 medications for phaco-only eyes vs 0.2 for CyPass eyes [P < 0.001]).
Looking Ahead
In addition to representing an area of considerable excitement in ophthalmology, MIGS devices are also an area of active investigation. There is good evidence for their efficacy when used in appropriately selected patients, although additional studies are necessary to evaluate the long-term efficacy and safety of these devices on a large scale, as made clear by the recent decision by Alcon regarding CyPass. In the meantime, new stent devices are likely to come to market in the future, such as the iStent Supra and the InnFocus MicroShunt, for which clinical trials for FDA approval are ongoing.
1. Samuelson TW, Katz LJ, Wells JM, et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459-467.
2. Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: Two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339-1345.
3. Arriola-Villalobos P, Martínez-de-la-Casa JM, Díaz-Valle D, et al. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: A long-term study. Br J Ophthalmol. 2012;96:645-649.
4. Fea AM, Consolandi G, Zola M, et al. Micro-bypass implantation for primary open-angle glaucoma combined with phacoemulsification: 4-year follow-up. J Ophthalmol. 2015;2015:795357.
5. Katz LJ, Erb C, Guillamet A, et al. Prospective, randomized study of one, two, or three trabecular bypass stents in open-angle glaucoma subjects on topical hypotensive medication. Clin Ophthalmol. 2015;9:2313-2320.
6. Vold SD, Voskanyan L, Tetz M, et al. Newly diagnosed primary open-angle glaucoma randomized to 2 trabecular bypass stents or prostaglandin: Outcomes through 36 months. Ophthalmol Ther. 2016;5(2):161-172.
7. Fea AM, Belda JI, Rekas M, et al. Prospective unmasked randomized evaluation of the iStent inject versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014;8:875-882.
8. Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: The HORIZON study [published online ahead of print June 23, 2018]. Ophthalmology.
9. Schlenker MB, Gulamhusein H, Conrad-Hengerer I, et al. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124(11):1579-1588.
10. Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25-36.
11. Vold S, Ahmed IK, Craven ER, et al. Two-year COMPASS trial results: Supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123(10):2103-2112.






