CASE PRESENTATION
A 42-year-old woman is referred by her primary eye care provider for a cataract surgery evaluation. The patient states that her distance and near vision has decreased during the past several years. Her occupation requires extensive computer use, but she is no longer able to see the screen clearly even with the aid of glasses. She has researched cataract surgery and implant technology extensively and requests a nondiffractive extended depth of focus (EDOF) IOL so that she can regain her distance and intermediate vision.
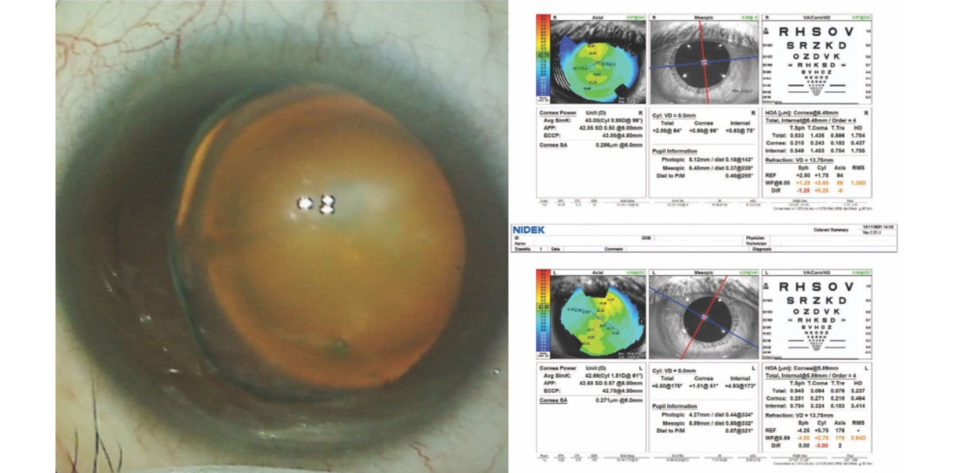
On examination, the patient’s BCVA is 20/30 OD and 20/40 OS. The IOP is normal in both eyes. There is no evidence of an afferent pupillary defect. A slit-lamp examination finds a white and quiet conjunctiva in each eye. Both corneas are clear, and the anterior chambers are deep and quiet. Slight decentration of each pupil is evident. An examination of the crystalline lenses finds 3+ nuclear sclerotic changes and cortical changes in each eye. A dilated fundus examination finds healthy optic nerves and retinas, but temporal subluxation of both lenses is evident (Figure 1). On questioning, the patient states, “Oh, yes, I think I have something called ectopia lentis.” Her topography and biometry are shown in Figures 2 and 3, respectively.
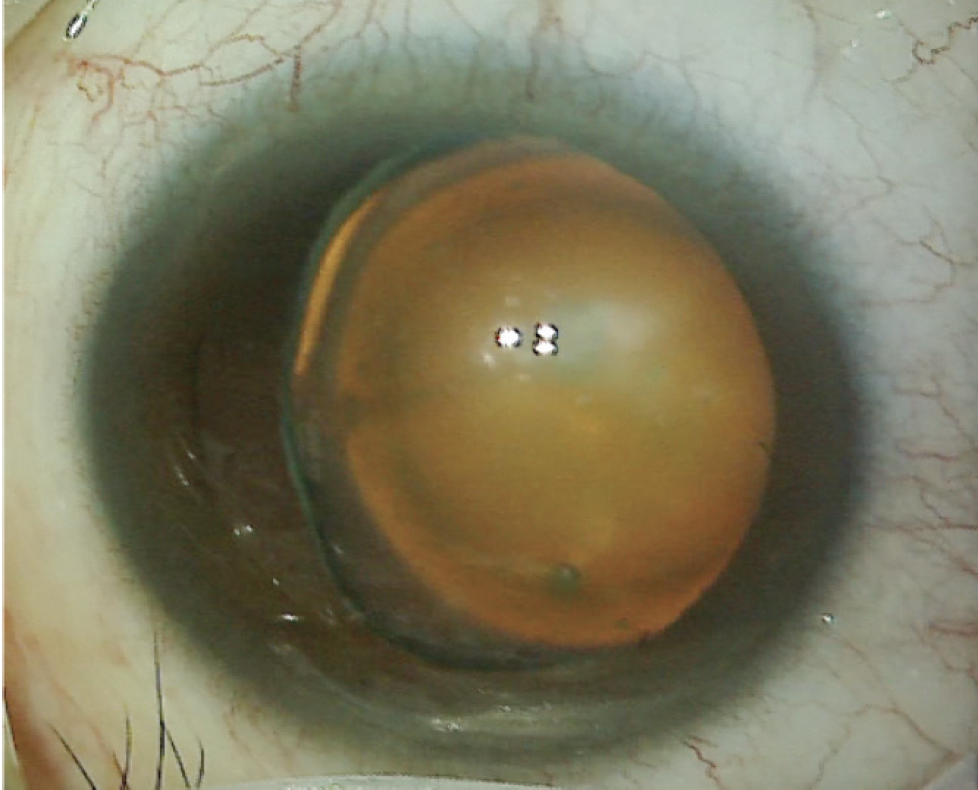
Figure 1. The left eye has a cataract and exhibits temporal subluxation of the crystalline lens.
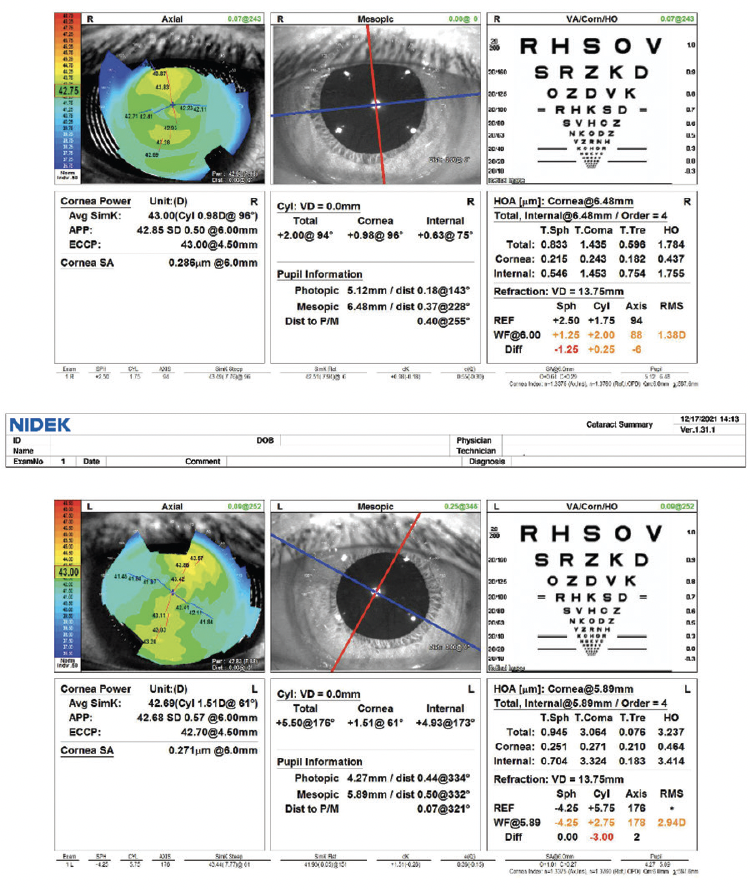
Figure 2. Computerized corneal topography of the right and left eyes.
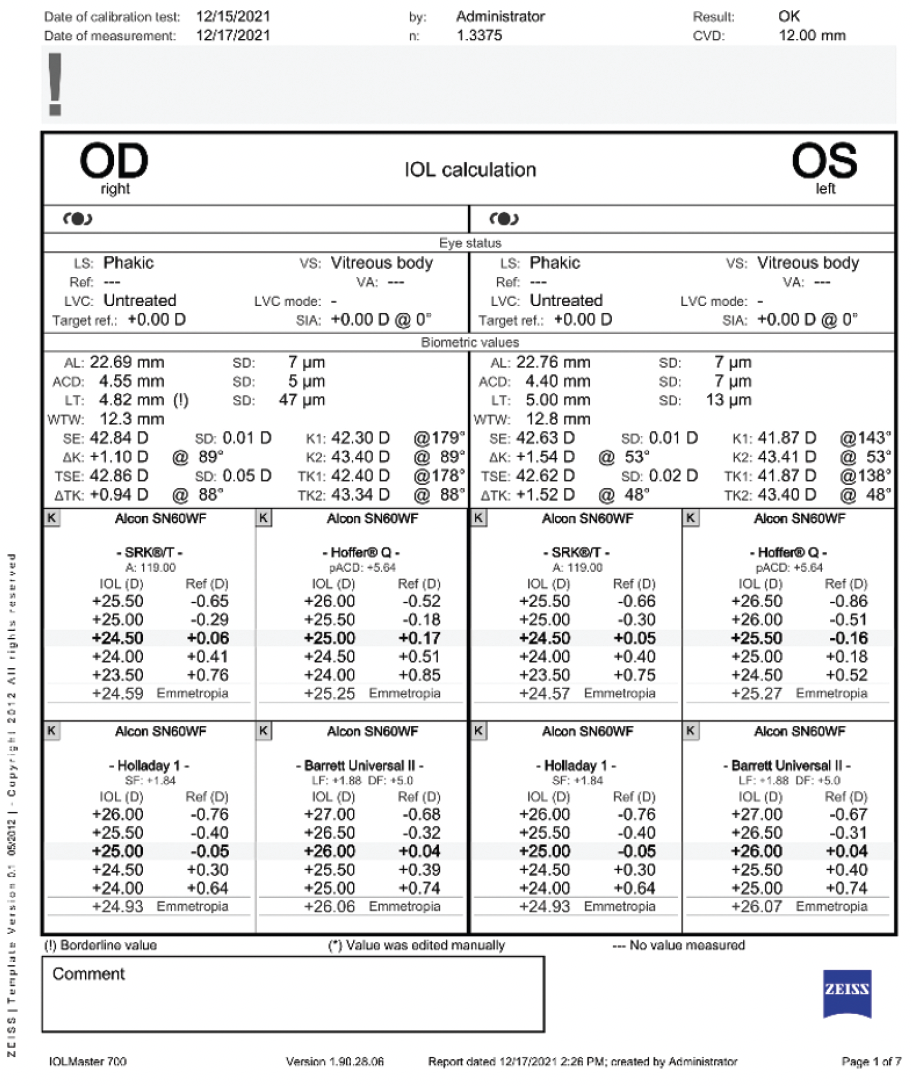
Figure 3. Biometry for both eyes.
Figures 1–3 courtesy of Brandon D. Ayres, MD
How would you manage the patient’s expectations, and how would you approach surgery?
— Case prepared by Brandon D. Ayres, MD

J. MORGAN MICHELETTI, MD
It is imperative that the patient understand the variables that may be encountered during and after surgery, including a dropped nucleus and late decentration of the IOL. I would ask if she has a history of connective tissue disorders, but given the data provided, I suspect this is a case of simple (ie, nonsyndromi) ectopia lentis, which often presents bilaterally.1
I would address the patient’s expectations and discuss the approaches that may fit her needs. I would recommend a contact lens trial to determine if monovision is a viable option. She cannot achieve better than 20/30 BCVA currently, but that could be because of high lenticular astigmatism due to ectopia lentis.2 She is motivated to succeed with an EDOF IOL. If she understands the potential risks of the technology in her situation, including an increased risk of decentration, a potential need for additional surgery (eg, LASIK or PRK enhancement, IOL explantation, intrascleral haptic fixation), and a possible change in plans intraoperatively, then I would consider attempting to implant a nondiffractive toric EDOF IOL with scleral fixation of the capsular bag–IOL complex.
IOL calculations would be performed with multiple formulas, including ones that do not depend on preoperative measurements of the anterior chamber depth for the estimated lens position. Accurate tracking, moreover, of the swept-source OCT image obtained with the IOLMaster 700 (Carl Zeiss Meditec) would be confirmed. Intraoperative aberrometry might also be useful.
The pupil of the left eye appears to dilate well (Figure 1). A laser capsulotomy (Figure 4) would therefore be performed to minimize stress on the zonules, and a capsular tension segment (CTS) would be inserted in the area of the zonular dialysis (Figure 5). Next, a zonule-friendly nuclear disassembly technique such as horizontal chop would be implemented followed by bimanual irrigation and aspiration, IOL insertion, and the suture fixation of one or more CTSs (Figures 6 and 7). A standard capsular tension ring (CTR) or a model with an eyelet would be inserted; the type of device and the timing of its placement would depend on the intraoperative findings.
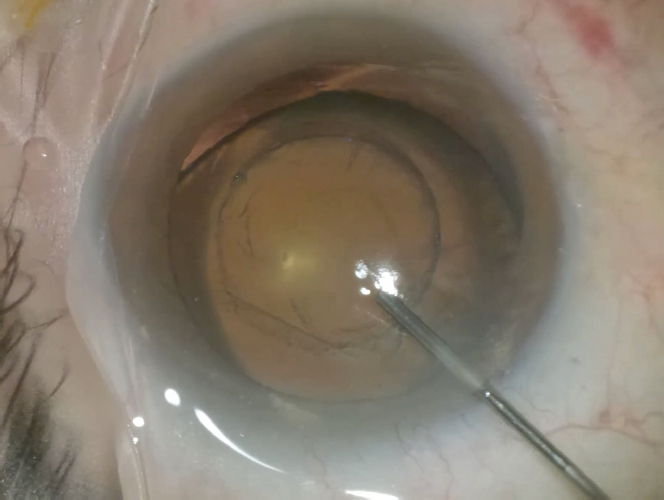
Figure 4. A laser capsulotomy is created in an eye with a
subluxated crystalline lens.
Figures 4–7 courtesy of J. Morgan Micheletti, MD
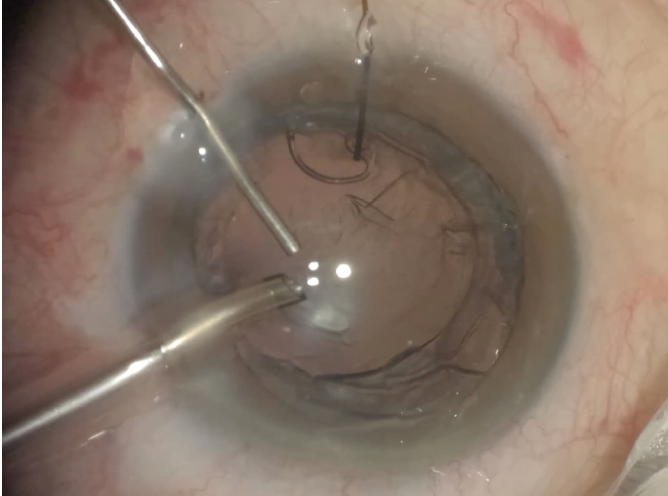
Figure 5. A CTS and iris hook are used to stabilize the capsular bag before horizontal chop and phacoemulsification are performed.
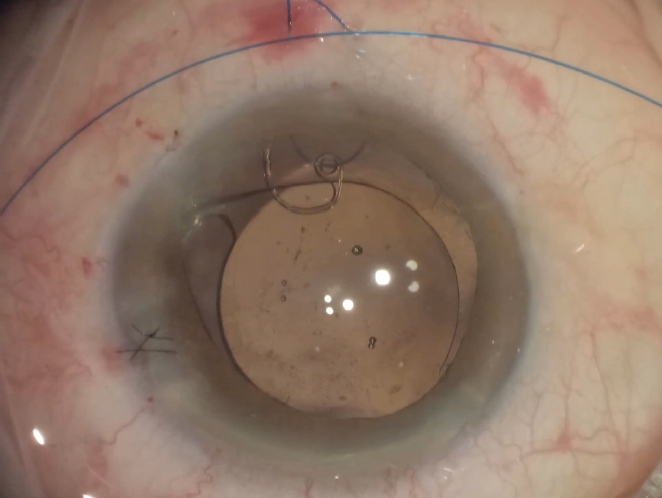
Figure 6. A CTS is secured to the sclera with flanges by means of a horizontal belt loop technique using a 6-0 polypropylene suture.
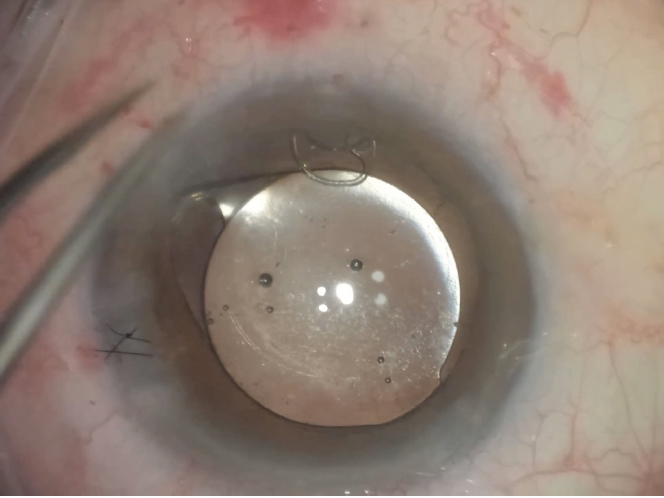
Figure 7. Final positioning of the IOL with a sutured CTS. Pupillary cerclage is then performed.
To paraphrase the poet Robert Burns, the best laid plans of mice and men often go awry. I would have backup plans prepared for surgery. If at any point it appears that the bag or IOL may not remain in a suitable position for success with a toric or EDOF IOL, then a monofocal IOL might be implanted because it can tolerate mild decentration. The patient’s astigmatism could then be treated postoperatively with a corneal refractive procedure. If the capsular bag cannot be salvaged, intrascleral haptic fixation of a Light Adjustable Lens (RxSight) would be performed. All these possibilities would be discussed with the patient preoperatively, and she should express comfort with the possible outcomes before surgery is undertaken.

BERNARDO DE PADUA SOARES BEZERRA, MD, FICO, FRANZCO
Management of the patient’s expectations is a priority in this situation. It is important she understand that surgery will be complex. She currently has reasonable visual acuity—still within driving range—but is likely struggling with her intermediate vision at work because of a reduction in accommodation secondary to zonular fragility. A systemic cause for her ectopia lentis must be excluded. A thorough family history would be obtained. Marfan syndrome and homocystinuria are the most common causes of heritable ectopia lentis and must be excluded.3,4 These conditions can be life-threatening, so a multidisciplinary approach would be preferable if the patient has either one.
The zonular defect is larger than 6 clock hours, so a referral to a posterior segment surgeon would be a reasonable option. An anterior approach would require addressing the zonular instability with a combination of a CTR and CTSs or a sutured Cionni CTR (Morcher). Capsular hooks could aid IOL positioning. A dispersive OVD would be instilled and reinjected as needed to prevent vitreous loss and sudden anterior chamber decompression. Key points are not to rush surgery and to remember that the anterior chamber is considerably deeper than is typical, so instruments and incisions should be positioned to facilitate access.
The ideal placement of the IOL would be in the bag. A monofocal lens would be my preference. I would implant an EDOF, toric, or toric EDOF IOL only if the capsular bag appears to be stable. If the bag is lost or zonular support is insufficient, then I would consider implanting a secondary IOL.
Extended chair time and comprehensive counseling could help the patient become a partner in her care.

DAGNY C. ZHU, MD
Given the bilateral temporal involvement, deep anterior chambers, large myopic shift, and high angle kappa (OS > OD), the patient likely has diffuse, progressive zonular weakness possibly secondary to an underlying systemic disease such as Marfan syndrome. Most important is to ensure that she has realistic expectations because it may not be possible to implant her IOL of choice. That said, I would try to deliver the best refractive outcome possible.
After the administration of a sub-Tenon block and a triamcinolone-assisted anterior vitrectomy, the capsule would be painted with trypan blue dye under an OVD. A capsulorhexis centered on the lens would be created, and countertraction would be applied as needed with a second instrument. An alternative would be to use the Zepto Capsulotomy System (Centricity Vision) to facilitate capsulotomy creation and centration.
Phacoemulsification and cortical removal would be performed after the placement of capsular hooks. A CTR and a scleral-fixated CTS would then be placed in the area of zonular weakness using the Canabrava double-flanged technique and 5-0 PTFE sutures.5 If the bag appears to be well centered and stable, a toric EDOF IOL (Clareon Vivity, Alcon) would be implanted and centered by adjusting the polypropylene flange. If centration of the capsular bag remains a concern, one option would be to implant a monofocal IOL (Tecnis Eyhance, Johnson & Johnson Vision) and implement a mini-monovision strategy. The implantation of a monofocal Light Adjustable Lens with a mini-monovision strategy is an alternative, but the silicone material of the lens could become problematic if the patient needs retinal surgery in the future. Scleral fixation of a three-piece CT Lucia IOL (Carl Zeiss Meditec) using the Yamane technique6 would be my last resort.

WHAT I DID: BRANDON D. AYRES, MD
Cataract surgery was required if the patient was to have any hope of experiencing an improvement in vision. The obvious zonulopathy, however, significantly increased the risk of vitreous loss and intraoperative complications, including lens subluxation. Any compromise in the integrity and position of the capsular bag would limit the possibility of implanting a presbyopia-correcting IOL and reduce the accuracy of biometry by changing the effective lens position.
The challenges, complexity, and risks of surgery were discussed with the patient. Initially, a monofocal IOL was recommended, but after persistent encouragement from the patient, a nondiffractive EDOF IOL was selected with the understanding that, if the capsular bag could not be used for implantation, scleral fixation of a monofocal IOL would be performed. She was then scheduled for cataract surgery on the left eye.
Surgical procedure Because scleral incisions would be necessary to fixate the capsular bag or for a vitrectomy, a retrobulbar block with sedation was used for anesthesia. A capsulorhexis was initiated with a cystotome and microforceps. As the capsulorhexis was created, additional limbal paracentesis incisions were made. Three nasal capsular support hooks were placed to facilitate better centration of the lens and safe completion of the capsulorhexis (Figure 8). The nuclear material was removed with phacoemulsification. Special care was taken to minimize shallowing of the anterior chamber by placing an OVD before the phaco tip was removed. Cortical removal was facilitated with the use of a bimanual I/A handpiece.
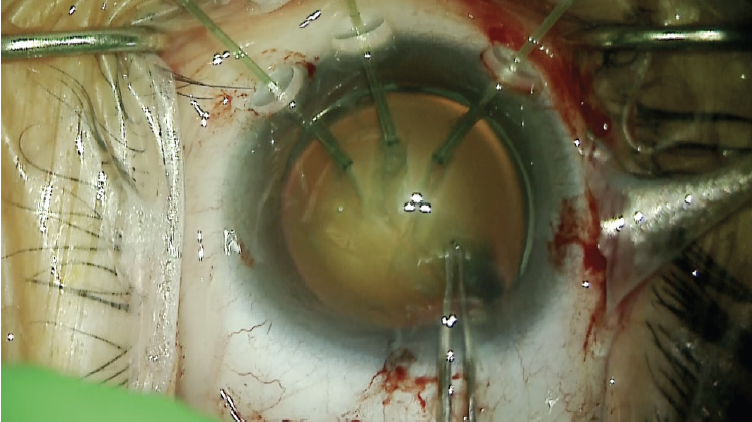
Figure 8. Capsular support hooks act as temporary support
to allow centration of the capsular bag and completion of
the continuous curvilinear capsulorhexis.
Figures 8–12 courtesy of Brandon D. Ayres, MD
After successful removal of the lens material, a 12-mm CTR was carefully inserted into the capsular bag, and the capsular support hooks were removed through the main incision (Figure 9).
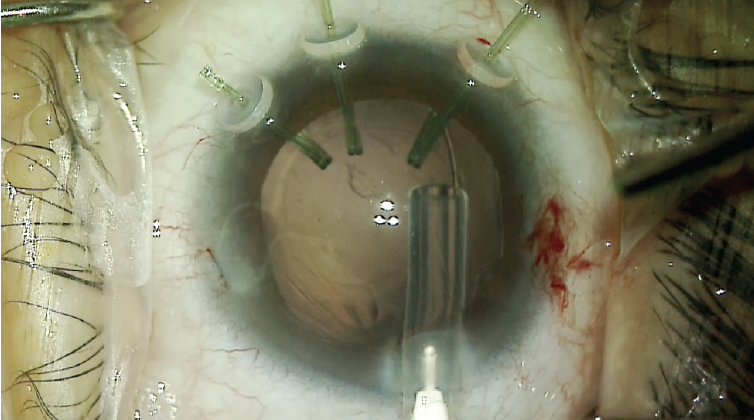
Figure 9. A CTR is inserted while the capsular hooks are in place. Once the CTR has been positioned, the capsular hooks are removed.
A nasal conjunctival peritomy was created, and two 1-mm scleral incisions were made 2 mm posterior to the limbus entering the sulcus space. One incision was made above and the other below the horizontal meridian. A size CV-8 PTFE suture (Gore-Tex, W.L. Gore & Associates; off-label use) was laced through the eyelet of a CTS. The ends of the suture were placed in the anterior chamber and then externalized through the two scleral incisions. The CTS was placed in the capsular bag, and a slipknot was tied on the scleral surface and titrated to center the capsular bag (Figure 10). For safety, a second CTS was placed on the temporal side using the same technique. Once the capsular bag was well positioned, the PTFE knots were buried in the scleral incisions (Figure 11).
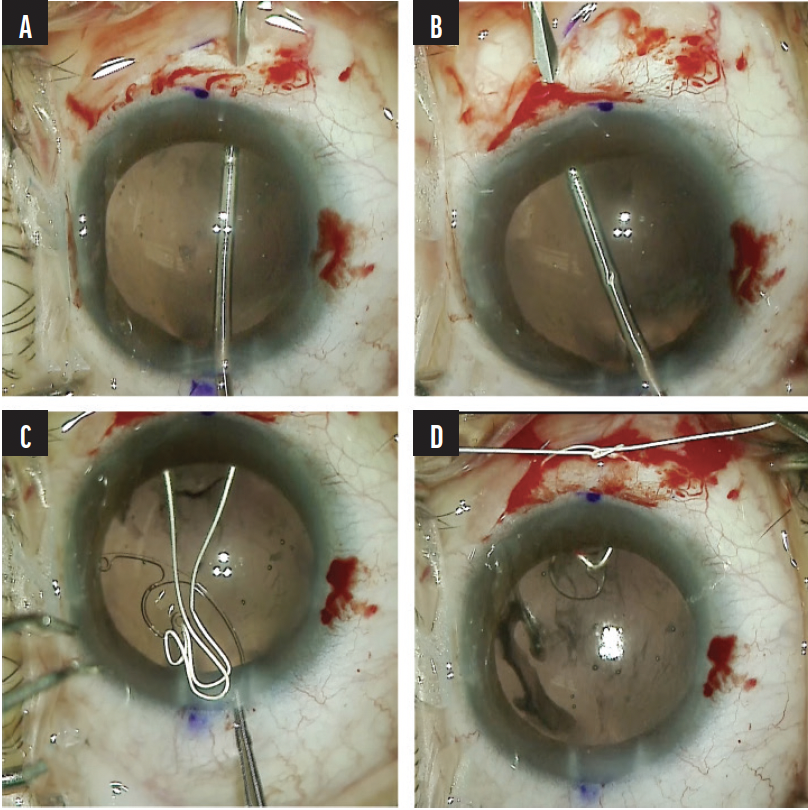
Figure 10. A scleral incision is created 2 mm posterior to the limbus entering the sulcus space. Note the gentian violet mark at the limbus delineating the horizontal meridian (A). A second scleral incision is created above the horizontal meridian 2 mm posterior the limbus entering the sulcus space (B). A PTFE suture is laced through the eyelet of a CTS, and the ends of the suture are externalized through the scleral incisions (C). A slipknot is tied, and the tension is titrated to center the capsular bag (D).
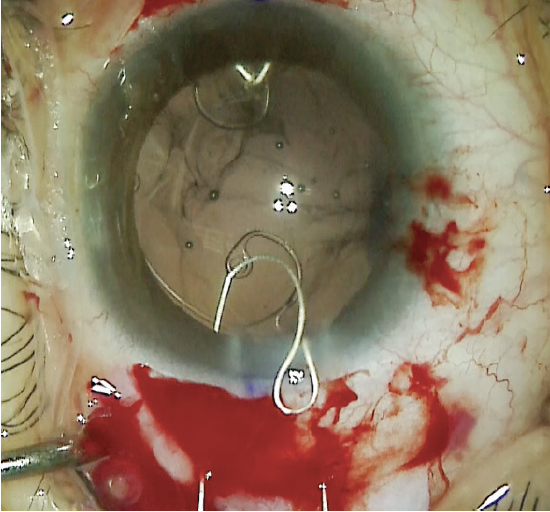
Figure 11. A CTS is placed in the temporal zone to provide additional capsular support.
A toric nondiffractive EDOF IOL was inserted into the capsular bag and carefully rotated to the steep axis. OVD was then carefully removed using bimanual irrigation and aspiration. The corneal incisions were hydrated or sutured to create a watertight closure. The conjunctival incisions were closed with an 8-0 polyglactin suture (Vicryl, Ethicon). At the conclusion of surgery, carbochol was instilled in the anterior chamber to induce miosis and help control IOP during the early postoperative period (Figure 12).
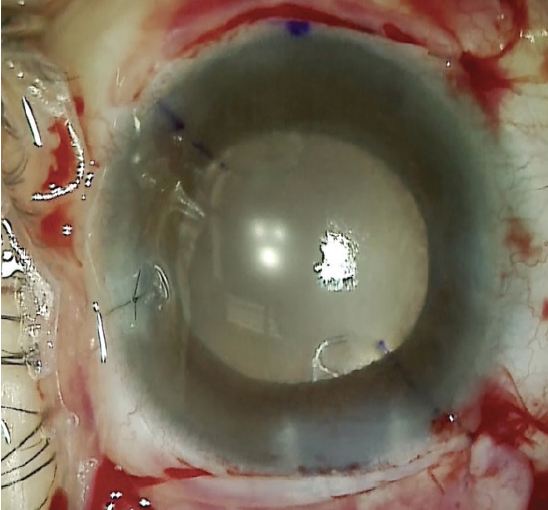
Figure 12. The immediate postoperative appearance of the eye.
Postoperative course. One day after surgery, the patient’s UCVA was 20/60 OS, and her pinhole visual acuity was minimally better. The IOP was 10 mm Hg OS. A slit-lamp examination found moderate corneal stromal edema, 2+ cell in the anterior chamber, a miotic pupil, and a well-centered IOL. The patient continued administering antibiotic and steroid drops four times daily until she returned at 1 week.
At that visit, her uncorrected distance visual acuity was 20/30 OS. She was able to read her mobile phone when it was held at arm’s length but needed a +1.75 D add for comfortable near reading.
The patient has requested cataract surgery on her right eye, but this time she wants … a trifocal IOL.
1. Chandra A, Aragon-Martin JA, Hughes K, et al. A genotype-phenotype comparison of ADAMTSL4 and FBN1 in isolated ectopia lentis. Invest Ophthalmol Vis Sci. 2012;53(8):4889-4896.
2. Nelson LB, Maumenee IH. Ectopia lentis. Surv Ophthalmol. 1982;27(3):143-160.
3. Wakita M, Ryu E, Nakayasu K, et al. Statistical analysis of Marfan’s syndrome. Nippon Ganka Gakkai Zasshi. Article in Japanese. 1989;93(6):682-690.
4. Neely DE, Plager DA. Management of ectopia lentis in children. Ophthalmol Clin North Am. 2001;14(3):493-499.
5. Canabrava S, Canêdo Domingos Lima AC, Ribeiro G. Four-flanged intrascleral intraocular lens fixation technique: no flaps, no knots, no glue. Cornea. 2020;39(4):527-528.
6. Yamane S, Sato S, Maruyama-Inoue M, Kadonosono K. Flanged intrascleral intraocular lens fixation with double-needle technique. Ophthalmology. 2017;124(8):1136-1142.