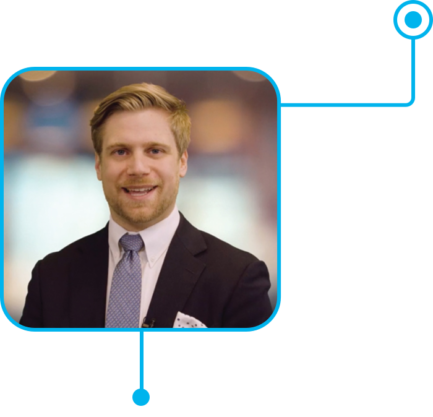
As a cataract, refractive, and anterior segment surgeon at Berkeley Eye Center in Houston, Morgan Micheletti, MD, knows that patients today require good vision at all distances. And while glasses have been a burden that his patients have dealt with for many years, times are now changing. With the Clareon® Vivity® and Clareon® PanOptix® Trifocal IOLs, bolder vision is now possible.1,2 Dr. Micheletti believes that it takes boldness to pioneer something new, and he appreciates the technology that Alcon has brought to the field so that his patients can live boldly as well.

“Patients come back saying, 'I can't believe I don't need glasses. My world has changed so much, and it's truly amazing. Several of them have gotten very emotional with their new vision.”*
“Spectacle independence enables my patients to really enjoy everything life has to offer.”*

“Many of my patients are trial attorneys and need to be able to quickly refer to notes, then look back up and make contact with the judge or the jury. These rapid transitions are possible with PanOptix®.”2


*80.5% of PanOptix IOL patients reported that they never needed glasses post cataract surgery with the PanOptix Trifocal IOL. Survey question asked, “How often have you needed glasses within the past 7 days?”
IMPORTANT PRODUCT INFORMATION - Clareon® Panoptix® and Vivity® Family of IOLs
CAUTION: Federal (USA) law restricts this device to the sale by or on the order of a physician.
INDICATIONS
The Clareon® PanOptix® Trifocal Hydrophobic IOL, Clareon® PanOptix® Toric, Clareon® Vivity® Extended Vision Hydrophobic Posterior Chamber IOL and Clareon® Vivity® Toric IOLs are indicated for visual correction of aphakia in adult patients following cataract surgery. In addition, the Clareon® Toric IOLs are indicated to correct pre-existing corneal astigmatism at the time of cataract surgery. The Clareon® PanOptix® lens mitigates the effects of presbyopia by providing improved intermediate and near visual acuity, while maintaining comparable distance visual acuity with a reduced need for eyeglasses, compared to a monofocal IOL. The Clareon® Vivity® lens mitigates the effects of presbyopia by providing an extended depth of focus. Compared to an aspheric monofocal IOL, the lens provides improved intermediate and near visual acuity, while maintaining comparable distance visual acuity. All of these IOLs are intended for placement in the capsular bag.
WARNINGS/PRECAUTIONS: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting a lens in a patient with any of the conditions described in the Directions for Use labeling. Physicians should target emmetropia, and ensure that IOL centration is achieved.
For the PanOptix® Toric and Vivity® IOLs, the lens should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation.
For the Clareon® PanOptix® IOL, some visual effects may be expected due to the superposition of focused and unfocused multiple images. These may include some perceptions of halos or starbursts, as well as other visual symptoms. As with other multifocal IOLs, there is a possibility that visual symptoms may be significant enough that the patient will request explant of the multifocal IOL. A reduction in contrast sensitivity as compared to a monofocal IOL may be experienced by some patients and may be more prevalent in low lighting conditions. Therefore, patients implanted with multifocal IOLs should exercise caution when driving at night or in poor visibility conditions. Patients should be advised that unexpected outcomes could lead to continued spectacle dependence or the need for secondary surgical intervention (e.g., intraocular lens replacement or repositioning). As with other multifocal IOLs, patients may need glasses when reading small print or looking at small objects. Posterior capsule opacification (PCO), may significantly affect the vision of patients with multifocal IOLs sooner in its progression than patients with monofocal IOLs.
For the Clareon® Vivity® IOL, most patients implanted with the Vivity® IOL are likely to experience significant loss of contrast sensitivity as compared to a monofocal IOL. Therefore, it is essential that prospective patients be fully informed of this risk before giving their consent for implantation of the Clareon® Vivity® IOL. In addition, patients should be warned that they will need to exercise caution when engaging in activities that require good vision in dimly lit environments, such as driving at night or in poor visibility conditions, especially in the presence of oncoming traffic. It is possible to experience very bothersome visual disturbances, significant enough that the patient could request explant of the IOL. In the parent AcrySof® IQ Vivity® IOL clinical study, 1% to 2% of AcrySof® IQ Vivity® IOL patients reported very bothersome starbursts, halos, blurred vision, or dark area visual disturbances; however, no explants were reported.
Prior to surgery, physicians should provide prospective patients with a copy of the Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with these IOLs.
ATTENTION: Reference the Directions for Use labeling for each IOL for a complete listing of indications, warnings and precautions.
1. Vivity DFU.
2. PanOptix DFU.
Dr. Morgan Micheletti is a paid Alcon consultant. PanOptix and Vivity are trademarks for Alcon. All other brand/product names are the trademarks of their respective owners.
© 2022 Alcon Inc. 7/22 US-CPO-2200063