CASE PRESENTATION
An 81-year-old monocular man presents for a cataract evaluation. The patient states that he lost the vision in his left eye due to a retinal detachment and says that, since that time, he has been concerned about having surgery of any kind on his right eye. Worry about the potential complications of cataract removal prevented him from seeing an eye doctor for several years. He also recalls being told in the past never to have surgery on his right eye.
The patient reports a significant decline in vision in the right eye during the past 1 to 2 years. He says that he can no longer drive and that this lack of independence compelled him to visit his local eye doctor for an evaluation. After that examination, the patient was told that his cataract was too advanced to be removed safely at that practice, and he was referred for specialty care.
Upon examination, visual acuity is no light perception in the left eye and counting fingers in the right eye, with no improvement noted on pinhole refraction. IOP is 17 mm Hg OU. A slit-lamp examination of the right eye shows a normal conjunctiva, a clear cornea, a moderately shallow anterior chamber, and a normal iris. The lens exhibits 4+ brunescent nuclear sclerosis but no sign of phacodonesis (Figure 1). The cataract prevents direct visualization of the retina, but a B-scan ultrasound is normal.
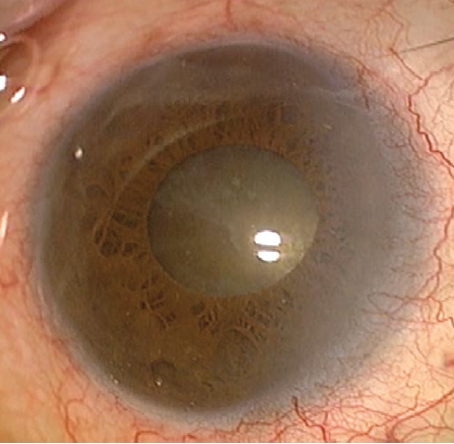
Figure 1. A preoperative examination of the right eye shows a relatively normal anterior segment and a dense nuclear sclerotic cataract. The pupil dilates relatively well with cycloplegic drops.
Figures courtesy of Brandon D. Ayres, MD

THOMAS BOLAND, MD
Cases such as this one are unfortunately all too common, and they are the reason why I encourage monocular patients to consider cataract surgery sooner rather than later. My first step would be to establish a rapport with this patient and reassure him that the risk involved with surgery, even with an advanced cataract, is low and that he should expect to recover good vision very shortly after the procedure.
In the OR, I would first examine the red reflex and the pupil size under the operating microscope. A pupil of the size shown in Figure 1 may be adequate for routine cataract surgery but not for a difficult monocular surgery. After making the incision, I would instill a cohesive OVD to create space and permit the placement of a pupil expansion device. After the ring was in place and the pupil had enlarged to 6 to 7 mm, I would reevaluate the red reflex and my ability to proceed with the capsulorhexis. If visibility is inadequate, the cohesive nature of the OVD should facilitate its removal so that the capsule can be stained with trypan blue dye. The chamber can then be reformed with a dispersive OVD, which will offer superior corneal protection for the high phaco energy that I would expect to use.
I would create a capsulorhexis of generous size to decrease the risk of capsular compromise while I work in the periphery because I often have to extend peripherally to split the lens. I would assess the composition of the lens during hydrodissection. A truly brunescent lens has very little cortical material, and the posterior portion of the lens is generally difficult to crack with normal techniques. It may be beneficial to use a nuclear fragmentation device such as the miLoop (Carl Zeiss Meditec), which can fragment a lens into two or more sections before the phaco handpiece is introduced.
I would replenish the dispersive OVD before commencing phacoemulsification. If I had already sectioned the lens nucleus, I would start phacoemulsification in the quadrant setting and slowly remove the nucleus while using the lowest effective phaco energy setting and frequently adding OVD. There is often very little cortex to clean up at the end of cases such as this one, and IOL implantation may then be carried out.
I find that corneas are typically clear on postoperative day 1 and visual recovery is rapid.

BRENTON FINKLEA, MD
When planning surgery on eyes with far advanced brunescent cataracts, I consider the option of manual small-incision cataract surgery. This form of extracapsular cataract extraction does not involve the use of phacoemulsification, thereby avoiding the high levels of ultrasound energy required to break up a dense lens. This is particularly beneficial in eyes that may have comorbid endothelial disease.
For most manual small-incision cataract surgery cases, I use local anesthesia with a sub-Tenon or peribulbar block. I prefer a superior approach that begins with a conjunctival peritomy of 2 to 3 clock hours and is centered on the 12 clock position. I consider a temporal approach for eyes with comorbid glaucoma that may require a filtering procedure in the future. I create a partial-thickness frown-shaped sclerocorneal tunnel located 1.5 mm posterior to the limbus; the external diameter is approximately 7 mm, and the internal opening is slightly larger, sized to the diameter and density of the lens. I place iris hooks in eyes with poorly dilating pupils, such as in this case, because the diameter of internal pupillary expansion devices is not wide enough to permit passage of the dense cataract. Thanks to the self-sealing nature of the scleral tunnel, bimanual irrigation and aspiration through limbal paracenteses can be used for cortical removal and polishing. A three-piece monofocal IOL can be placed in the bag without folding using McPherson forceps.
Although this surgery can be performed without sutures, I sometimes place a single 10-0 nylon suture in the wound to help counteract the induction of against-the-rule astigmatism (approximately +1.25 D on average if no suture placed). Finally, given this patient’s history of retinal detachment in the fellow eye and no view of the retina preoperatively, I would perform a posterior examination without scleral depression 1 day after surgery to identify retinal pathology that should be addressed early in the postoperative course.


ABHAY R. VASAVADA, MS, FRCS; AND VAISHALI VASAVADA, MS
This patient’s history of retinal detachment in the fellow eye and the dense, brunescent cataract in his only seeing eye necessitate a thorough preoperative evaluation and a discussion with him and his family of potential surgical and postoperative challenges. In particular, discussion should cover the risk of postoperative corneal edema, which could delay visual recovery, and the rare possibility of a dropped nucleus because the status of the posterior segment and zonular integrity cannot be evaluated completely.
We would plan to perform phacoemulsification. An adequately sized capsulorhexis will be essential. To facilitate this step, we would not hesitate to stain the capsule with trypan blue dye. A judicious use of techniques and technology is crucial to success. It is important to recognize that cataract surgery involves distinct phases, each of which requires modification of phaco machine parameters. These phases include trenching (if performed), nuclear division, and removal of the fragments. When a cataract is extremely hard, we prefer to use the horizontal direct chop technique originally described by Nagahara. In general, we find that chopping techniques work better than divide-and-conquer in eyes such as this one.
The key is to divide the nucleus into as many completely separate fragments as possible. Cataracts such as this one are often leathery, so dividing the posterior plate can be a challenge. We would resort to multilevel chopping if necessary. This technique involves occluding the phaco tip peripherally first and then more centrally to achieve a good vacuum hold and effectively chop the nucleus from its periphery to its center and from a superficial to a deep level. In our experience, sequential occlusion allows more effective chopping without endangering the zonular apparatus.
Whatever technique for dividing the nucleus the surgeon uses, fragments must not be removed until the nucleus has been almost completely divided. Fluidic and ultrasound parameters must be borne in mind during fragment removal. We prefer to use torsional ultrasound because of its efficiency, particularly for dense cataracts. Whether torsional or longitudinal ultrasound is used, it will be important to use pulse or burst mode because a large amount of energy will be required for this cataract. Equally important will be to emulsify the fragments at the posterior plane, away from the corneal endothelium, in order to protect it. We would coat the endothelium with a dispersive OVD and replenish the OVD as needed.
Because the contralateral eye has a history of retinal detachment, we would evaluate the peripheral retina of the operative eye at regular intervals after surgery.

WHAT I DID: BRANDON D. AYRES, MD
During a long and candid preoperative discussion with the patient, I emphasized the risks of cataract surgery in an eye with a mature cataract such as his. I also carefully explained what to expect after surgery as far as recovery time and stated that, with no view to the retina, I could not give any guarantee regarding final recovery of vision. I assured the patient that I would do everything possible to minimize complications, and he elected to undergo cataract surgery.
The procedure was performed under topical anesthesia. To help maintain maximal dilation of the pupil, phenylephrine and ketorolac intraocular solution 1%/0.3% (Omidria, Omeros) was added to the irrigating fluid.
Visualization was poor, and trypan blue dye was used to stain the anterior capsule (Figure 2). A continuous curvilinear capsulorhexis was then created (target size, approximately 5 mm). Hydrodissection was carried out using balanced saline solution on a cannula. Phacoemulsification was used to make a central groove in the lens (Figure 3).
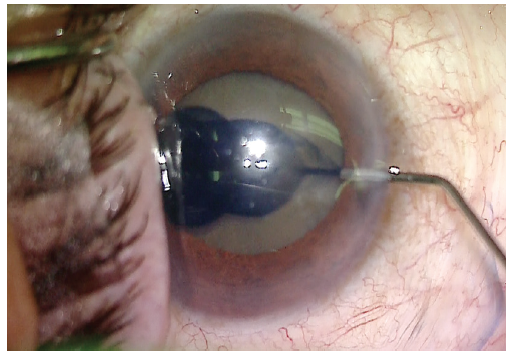
Figure 2. Trypan blue dye is used to stain the anterior capsule.
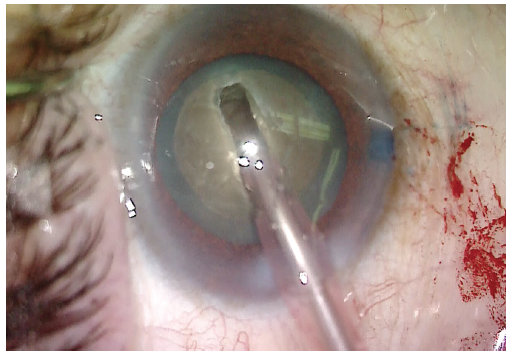
Figure 3. After the capsulorhexis and hydrodissection are performed, a deep groove is created in the center of the lens.
A small amount of OVD was placed under the anterior capsule to create a small space between the lens and the anterior capsule. A miLoop was tested and then carefully opened in the capsular bag and aligned with the deep groove in the lens. Once in position, the device was constricted, cleaving the lens into two pieces. I find that the deep groove helps to prevent rotation of the lens and facilitates division of the posterior plate of the lens as the miLoop is constricted (Figure 4). The lens was rotated 90º, and the device was deployed again, cleaving the lens into four quadrants (Figure 5).
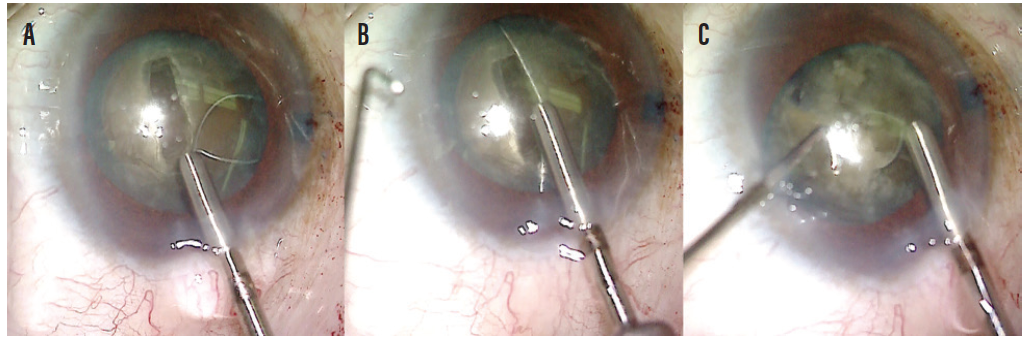
Figure 4. A miLoop is carefully expanded into the capsular bag (A). Once the device is open, the filament is aligned with the groove made by the phaco needle (B). The filament is constricted, cleaving the nucleus into two pieces and easily separating the posterior plate of the mature lens. A second instrument is used to facilitate the cleavage of the lens (C).
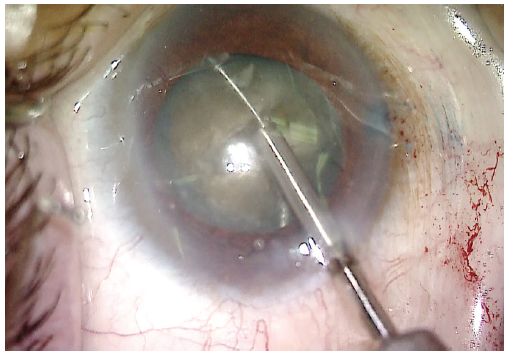
Figure 5. The lens is rotated 90º from the initial cleavage plane. A miLoop is once again used to cleave the lens, now into four quadrants.
Phacoemulsification was used to remove the quadrants. The prefragmentation with the miLoop made removal of the dense lens material significantly easier (Figure 6). Retained cortical material was removed with the I/A unit. More OVD was injected into the anterior chamber, and then a one-piece acrylic IOL was implanted (Figure 7). At the conclusion of the case, all OVD was removed from the anterior chamber and the incision was sealed.
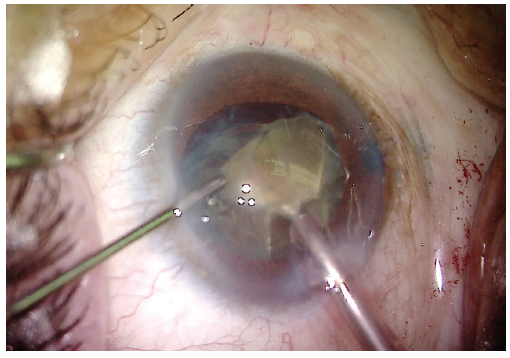
Figure 6. With the dense lens now divided into four quadrants and the dense posterior plate cleaved, the lens can be removed more easily with phacoemulsification.
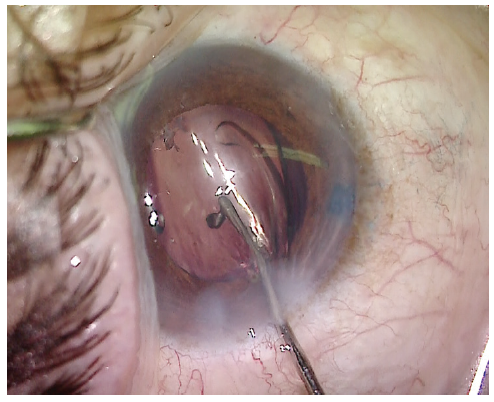
Figure 7. The IOL is implanted through a small incision.
In this case, the combination of phacoemulsification and mechanical lens cleavage allowed efficient small-incision cataract surgery with less ultrasound energy than might otherwise be expended on a lens of this density, thereby helping to protect the corneal endothelium (Figure 8). On postoperative day 1, UCVA was 20/70. Mild corneal stromal edema and endothelial folds were evident, and the IOP was normal. The patient was thrilled with the result, and UCVA was 20/40 at 1 week. Once his refraction stabilized, he was referred back to his primary eye care physician.
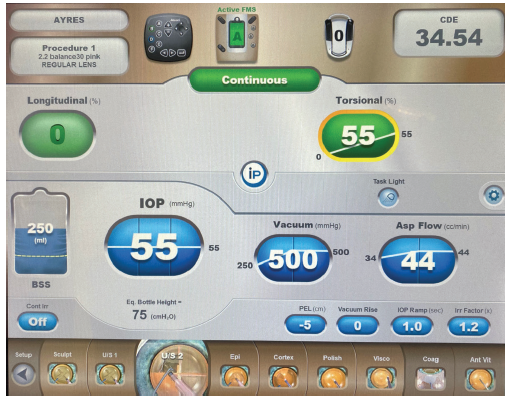
Figure 8. The phaco energy required was significantly lower than what Dr. Ayres would typically use for a lens of this density.