CASE PRESENTATION
A 42-year-old man presents with a complaint of increasing difficulty driving. A truck driver by trade, the patient reports no history of trauma or steroid use. BCVA is 20/40 OD and 20/30 OS, but it decreases to 20/200 OU on brightness acuity testing.
A central posterior polar cataract with 1+ to 2+ nuclear sclerosis is prominent in each eye (Figure 1). The rest of the examination is normal except that the patient is unable to undergo an indirect retinal examination without aggressive squeezing of his eyelids and tearing.
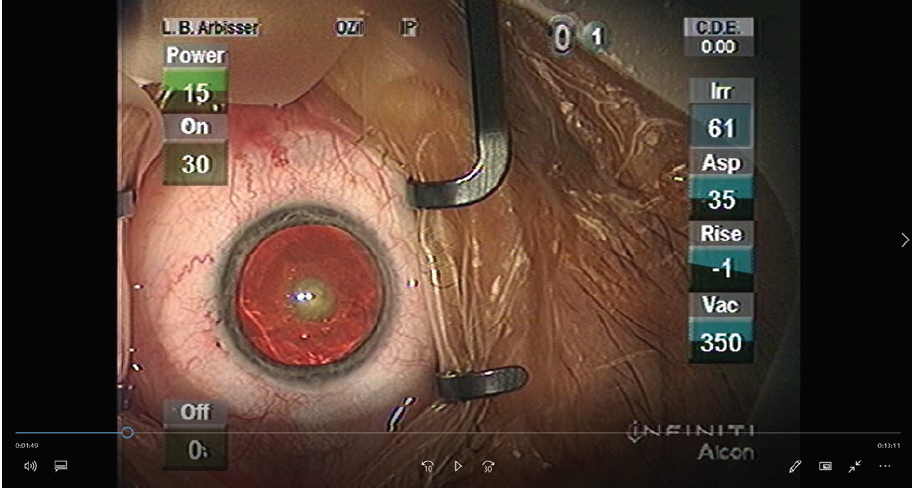
Figure 1. This patient had nearly symmetrical bilateral posterior polar cataracts. The left eye is shown.
How would you handle this case? Are there special precautions you would take, and are changes to the consent process, treatment, and technique indicated?
—Case prepared by Lisa Brothers Arbisser, MD

ZAINA AL-MOHTASEB, MD
I assume that there is a break in the posterior capsule in any eye with a posterior polar cataract. The consent process is important, and I make sure to emphasize to patients the heightened risk of complications and potential difficulty in placing an IOL intraoperatively. Ultrasound biomicroscopy or use of femtosecond laser imaging software can aid assessment of the posterior capsule prior to surgery.
In the OR, I would stain the capsule with trypan blue dye and perform a small capsulorhexis in case it becomes necessary to capture the optic of a sulcus-fixated IOL. I would then carefully perform hydrodelineation without hydrodissection to prevent hydrostatic pressure from creating or extending a posterior capsular tear. Hydrodelineation creates a bowl of epinucleus that acts as a mechanical cushion, protecting the posterior capsule during subsequent maneuvers. I would use a Nagahara chopper to cut the endonucleus into pieces without rotating the nucleus.
Before removing any instruments from the eye, I would inject an OVD to maintain the anterior chamber and prevent its collapse. I would use a dispersive OVD to viscodissect the epinucleus and cortex, especially in the area of concern to tamponade the vitreous. Then I would use bimanual irrigation and aspiration to remove the epinucleus and cortex. I would start in the periphery and move toward the area of concern. Even if the capsule is intact, I would avoid polishing it. After OVD placement, I would insert the IOL while taking care not to push centrally in the area of capsular abnormality.
I would be prepared to place the IOL in the sulcus and to perform an anterior vitrectomy if needed.

RUPERT MENAPACE, MD, FEBO
First, I would obtain anterior segment OCT imaging, which may clarify the integrity of the central posterior capsule (Figure 2). If it does not, then informed consent must explicitly state the increased risk of capsular tearing and possible need for alternative IOL fixation and/or vitrectomy and retinal complications. Specifically, there is an increased risk that a preexisting capsular defect will extend to the periphery.
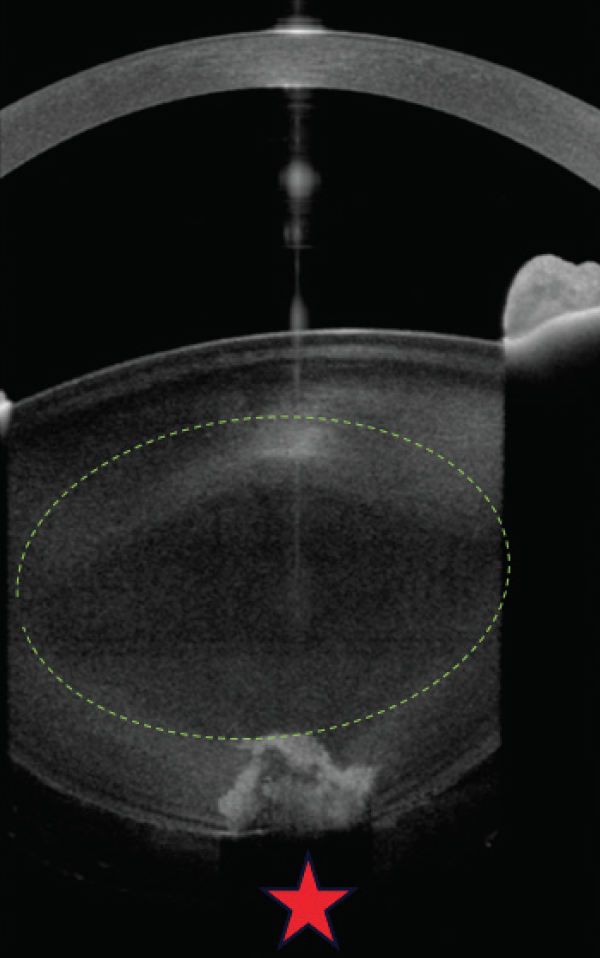
Figure 2. High-resolution anterior segment OCT shows an absent central posterior capsule, necessitating extreme surgical care after appropriate informed consent.
Courtesy of Rupert Menapace, MD, FEBO
Capsular cortical hydrodissection and pressure changes definitely must be avoided. I would use a femtosecond laser to create a 5-mm capsulotomy and to perform six-sector prefragmentation of the nucleus with a 700-µm posterior capsule offset and a central spiderweb pattern. After instilling a dispersive OVD, I would gently mobilize the gas-dissected sectors one by one with a chopper and manipulate them to occlude the phaco tip. I would employ low flow and vacuum and lower the bottle during phacoemulsification to avoid pressure changes and overpressurization of the chamber. Before changing instruments or hands during biaxial irrigation, I would lower the bottle and refill the anterior chamber with the OVD to keep the capsular diaphragm flattened out. I would carefully peel cortex from the periphery to the center. Then I would insert a gently unfolding one-piece open-loop IOL and, instead of using aspiration, irrigate the OVD out through the main incision.
If I were instead performing manual phacoemulsification, I would create a 5-mm capsulorhexis, perform gentle hydrodelineation, and disassemble the sequestered nucleus with slow-motion central sculpting.
If a radial posterior tear occurred, I would fixate a three-piece IOL in the sulcus and buttonhole the optic. If it were a minor tear, vitreous prolapse could be pushed back. If it were major, a prompt pars plana vitrectomy would be required.

NAVEEN K. RAO, MD
In the setting of a posterior polar cataract, it is important during the consent process to explain to patients that there is an elevated risk of posterior capsular tear and vitreous loss. It therefore may not be possible to place an IOL in the bag, which could affect the selection of a toric or multifocal design.
I would start surgery by making two paracentesis incisions, one on either side of the main incision. In cases such as this one, I perform hydrodelineation but not hydrodissection, and I do not spin the lens. Using the phaco tip through the main incision, I bowl out the endonucleus, which is typically soft. Next, I inject a dispersive OVD between the epinucleus and capsule to gently separate the posterior polar opacity from the capsule. If there is a capsular tear in the area of central adhesion, the dispersive OVD can flow through this tear into Berger’s space to help decrease the chance of vitreous prolapse. Then, using bimanual irrigation and aspiration through the paracentesis sites, I remove the epinucleus, starting peripherally and working toward the center. I find that bimanual irrigation and aspiration gives me better control and slows things down so that the central posterior polar opacity does not get sucked out unexpectedly.
After I remove the central posterior polar opacity with bimanual irrigation and aspiration, I carefully inspect the posterior capsule. If it is intact, I place a one-piece IOL in the bag. If there is a central round posterior capsular defect, an anterior vitrectomy may be required, but, if the anterior hyaloid face has not been disturbed and there is no vitreous prolapse, I would simply place a three-piece IOL into the sulcus and capture the optic through the anterior capsulorhexis. Optic capture can improve IOL centration, decrease the risk of iris chafing, and prevent vitreous prolapse into the anterior chamber.

WHAT I DID: LISA BROTHERS ARBISSER, MD
Congenital posterior polar cataracts evolve slowly but cause symptoms that seem out of proportion to Snellen acuity, often requiring patients to undergo surgery at a younger than normal age. Glare testing is essential to document true visual disability.
I discussed with this patient the nature of his posterior polar cataract, the rare risk that mild amblyopia would limit the potential for a 20/20 result, and the outsized risk of vision-threatening complications of surgery on an eye with this type of cataract.1 He opted to undergo phacoemulsification on his right eye first, followed by surgery on his left eye as and when appropriate. Together we chose to use a peribulbar block instead of topical anesthesia because of his extreme photophobia and uncontrolled blepharospasm.
I operated on the right eye first. From postoperative day 1, the patient was distinctly bothered by the vision in his left eye. After the need was documented, he returned for surgery in 2 weeks for bilateral rehabilitation.
In my opinion, a posterior polar cataract is not associated with agenesis of the posterior capsule, as is commonly thought, but is rather a cortical-capsular adhesion at the posterior pole without elasticity within the ring of adhesion. This predisposes the posterior capsule to rupture upon a contusion injury, which I believe is why the capsule is only rarely open preoperatively. It is important to diagnose and document a preexisting tear with anterior segment OCT if a patient has a history of trauma.
I schedule more time for these cases and prepare myself to be exceptionally patient because, when exposed to the usual forces of routine phacoemulsification, the posterior capsule is likely to rupture. With my approach, as described later and shown in the accompanying video, I achieved a streak of 63 consecutive posterior polar cataract surgeries before encountering my first broken capsule. Careful creation of a capturable anterior capsulorhexis or laser capsulotomy provides a fail-safe in the event of a ruptured posterior capsule. This fail-safe is a core principle of mine in any case, but it is especially important when the likelihood of rupture is increased. If a tear in the posterior capsule occurs, because it will be central, it can be converted to a true posterior continuous curvilinear capsulorhexis if recognized early and no force is applied to extend it peripherally. Any form of optic capture—anterior, reverse, or posterior—can be used as appropriate, so a three-piece lens should be available as a backup.
The first principle of my approach is to avoid deepening or shallowing of the anterior chamber until the adhesion is released. This means not overfilling the anterior chamber, lowering the bottle (or forced-infusion IOP), entering the eye with the phaco handpiece set to footpedal position zero in an OVD-filled environment, and lifting the iris off the peripheral capsule before engaging footpedal position 1. Infusion is then increased to normal parameters. This maneuver avoids retropulsion of the iris (reverse pupillary block) and resultant overdeepening of the anterior chamber with stretching of the adhesion and potential capsular rupture. Of course, care must be taken to establish flow prior to initiating ultrasound to avoid wound burn. Before removing either the phaco or the I/A handpiece, I instill an OVD through the sideport incision to stabilize the anterior chamber and prevent its collapse.
The second principle is not to hydrodissect but only to hydrodelineate the endonucleus and to avoid rotation of the nucleus during disassembly maneuvers. Vertical chop avoids the forces of sculpting and is my go-to technique in every case, but I find it particularly useful in these eyes because the endonucleus can be removed in situ.
When the lens is soft, as it was in this eye, I use a Rosen splitter, which has no sharp point, to dig layer by layer toward the plaque as I aspirate the endonucleus with the phaco tip. Minimal if any ultrasound energy was required in this case. After freeing the lenticular material from its central adhesion, I can carefully remove peripheral epinucleus while guarding the central capsule, which may still be weak. There is often a cortical layer of adhesion located just peripheral to the plaque, so the adhesion will not completely release until the cortex is stripped away little by little centripetally. In this situation, sometimes subincisional epinucleus must be loosened with hydrodissection—acceptable now that the adhesion has been relieved—performed with a J cannula and removed with irrigation and aspiration rather than the phaco tip. Cortical stripping centripetally, clock hour by clock hour, progressively releases any attachments to places where the periphery of the pancake-like polar plaque is adherent.
Extreme care must be exercised during polishing of the posterior capsule. I use a Terry squeegee well lubricated with an OVD for this purpose. In the first eye of this patient, I was unable to achieve a visually clear capsule, so I performed a primary hyaloid-sparing posterior capsulotomy to avoid the necessity for an early Nd:YAG laser capsulotomy. I was able to polish the posterior capsule of the second eye so that it was clear and intact, as shown in the accompanying video (bit.ly/1CSCASE0420). Instead of wound-assisted IOL insertion, I prefer to enlarge the incision slightly so that there is no expulsive force on the intact but potentially weak posterior capsule during IOL implantation.
1. Foster GJL, Ayers B, Fram N, et al. Phacoemulsification of posterior polar cataracts. J Cataract Refract Surg. 2019;45(2):228-235.




