CASE PRESENTATION
A 23-year-old woman presents for a refractive surgery consultation. The patient has a history of high myopia. She states that she has blurry vision even when wearing glasses and says that she has been unable to be fit with contact lenses. She is highly motivated to undergo refractive surgery to improve her lifestyle and quality of vision.
On examination, visual acuity is -24.00 -0.75 x 095º = 20/60- OD and -22.50 -2.50 x 081º = 20/60 OS. Average keratometry readings are 41.00 D OU. Corneal thickness is 525 µm OD and 527 µm OS. Anterior chamber depth is 3.6 mm OU. Topographic and tomographic maps appear to be normal for both eyes (Figure 1), and the anterior segment in each eye is normal.
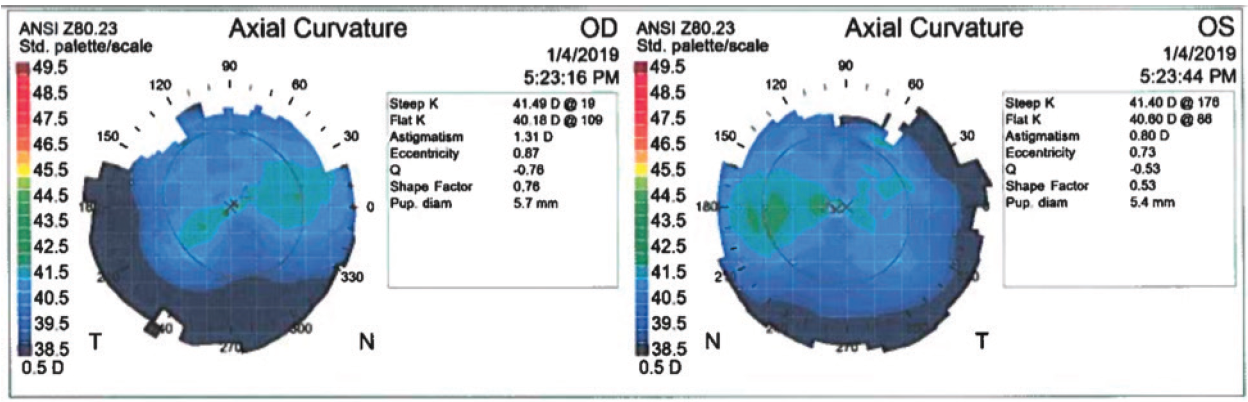
Figure 1. Topography appears to be normal in both eyes.
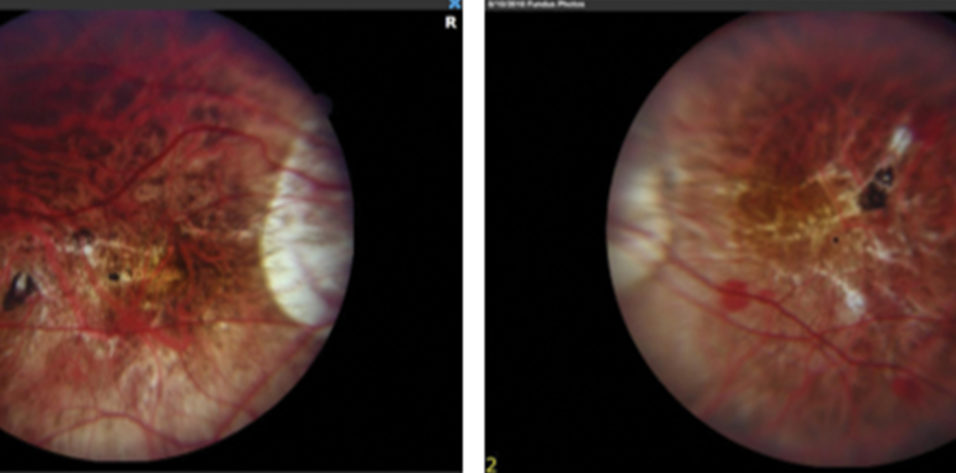
Fundus examination shows a myopic fundus in each eye. The right eye has a posterior vitreous detachment, and the macula has small lacquer cracks and a dot subretinal hemorrhage (Figure 2). The vitreous is attached in the left eye. OCT and fluorescein angiography (FA) show no subretinal fluid, neovascularization, or leakage.
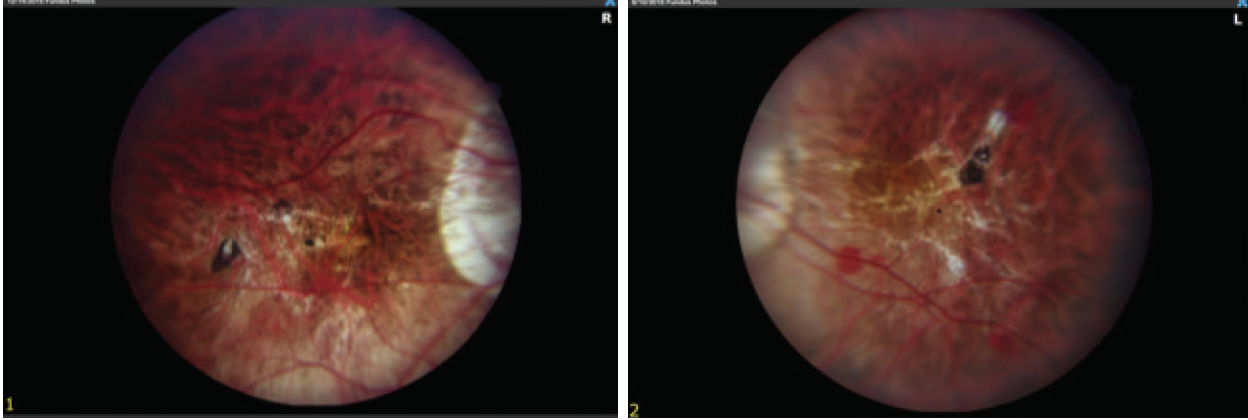
Figure 2. Myopic fundus appearance in both eyes shows tilted nerves and peripapillary atrophy. Lacquer cracks and a small retinal hemorrhage are visible in the fundus of the right eye (image on the left).
Which refractive options would you consider and ultimately recommend to this patient? Does decreased BCVA rule out elective refractive surgery for this patient? Do retinal findings in this case rule out elective refractive surgery for this patient? If you deem elective refractive surgery to be contraindicated, what other measures would you explore for this patient?
—Case prepared by William F. Wiley, MD
DAN Z. REINSTEIN, MD, MA(C), FRCSC, DABO, FRCO, FEBO
First, I would ensure that a retina specialist reviewed the posterior segment in full, including examination with scleral depression. The Visian ICL (STAAR Surgical) is a retina-safe option, and it is safer than refractive lens exchange for an extremely myopic eye in a young patient. Amblyopia is not a contraindication for refractive surgery, particularly if this patient is starting below the driving standard for best corrected distance visual acuity (CDVA). In addition to significantly decreasing her need for refractive correction, this patient would likely gain CDVA if she received a Visian ICL in each eye.1
The highest-powered model available corrects approximately -20.00 D (this model is not available in the United States). This patient could then be offered bioptics via a LASIK procedure. Sizing of the phakic IOL is critical to its long-term safety. The white-to-white standard from the 1990s is outdated and inferior to very high-frequency digital ultrasound (VHFDU) posterior chamber biometry.2-7 I use the ArcScan Insight 100 VHFDU (ArcScan) to directly measure a number of posterior chamber elements, including sulcus diameter, ciliary body diameter, and crystalline lens rise. Using VHFDU, in 50 consecutive eyes, I have been able to control lens separation (termed vault) to a mean of 506 µm with a standard deviation of less than 197 µm (range, 150–860 µm). More important, the chances of an outlier vault have been significantly reduced (D.Z.R., unpublished data).
Even if this patient did not undergo sequential corneal refractive surgery after receiving a Visian ICL in each eye, these IOLs should give her much more manageable options for further vision correction, thus greatly improving her quality of life. That said, however, contact lenses are inferior to corneal refractive surgery in terms of long-term risk, level of ocular comfort, and quality of night vision.8
LLEWELYN J. RAO, MD
As a vitreoretinal surgeon, I will refrain from commenting on refractive options. In highly myopic patients with retinal manifestations of myopic degeneration, I recommend consultation with a vitreoretinal specialist prior to refractive surgery. FA and OCT may be able to detect a myopic choroidal neovascular membrane, allowing prompt treatment and stabilization before any refractive surgical procedure. Additionally, a thorough inspection of the retinal periphery with scleral depression may detect retinal breaks or detachment, which I prefer to treat prior to an anterior segment procedure. Thankfully, this particular patient did not have any evidence of a choroidal neovascular membrane on examination, FA, or OCT. Of note, because of her high myopia, we were able to obtain clear FA and color photographs only by using an old camera system that allowed manual focusing adjustments.
Retinal findings can rule out elective refractive surgery or delay the procedure until the retina is considered to be stable. The goal is to achieve the best possible vision for patients, and that goal is compromised if vision is lost due to retinal pathology. Once a specialist clears a patient’s retina, the patient and his or her refractive surgeon can feel confident that everything possible was done to ensure the most successful outcome. If the retina decompensates postoperatively, the retina specialist already has a relationship with the patient as well as a good baseline evaluation, which is crucial to answering the question often raised in these situations: “Did refractive surgery cause my retinal problem?”
WHAT I DID: WILLIAM F. WILEY, MD
Given the patient’s age and prepresbyopic status, I recommended bilateral implantation of the Visian ICL followed by bilateral LASIK. We implanted the highest-powered lens model available in the United States, a -16.00 D Visian ICL with a diameter of 12.6 mm, in each eye. We knew that this IOL would correct only a portion of this patient’s refractive error and that she would likely need additional corneal refractive surgery to achieve maximal independence from glasses. We had contemplated using the Visian Toric ICL (STAAR Surgical) so as to decrease the patient’s astigmatism as well. Because we were planning bioptics (two-stage) surgery anyway, however, we felt that the astigmatism would be better treated with corneal refractive surgery, which would avoid the low but potential risk that the Visian Toric ICL would rotate from the intended position. Had an IOL model been available that would have treated the entirety of this patient’s spherical and cylindrical errors, our preference would have been a one-stage surgery involving implantation of that lens.
One month after bilateral ICL implantation, the laser peripheral iridotomies were patent, and the vault in each eye appeared to be approximately 500 µm. Manifest refraction was -7.00 -2.00 x 097º OD and -8.00 -2.00 x 095º OS, and CDVA was 20/40 OU.
Approximately 6 weeks after ICL implantation, the patient underwent bilateral LASIK for distance correction with the Allegretto Wave Eye-Q excimer laser system (Alcon). One week after surgery, uncorrected distance visual acuity (UDVA) was 20/40 OD and 20/40 OS, and bilateral UDVA was 20/30-. The patient was thrilled with the result. In the right eye, the manifest refraction was plano -0.75 x 120º, and BCVA was 20/40. In the left eye, the manifest refraction was +0.25 -0.75 x 050º, and BCVA was 20/40.
Before surgical intervention, we had an extensive discussion with the patient regarding her prior refractive amblyopia, and we emphasized that we did not expect her to see the 20/20 line after surgery. Throughout the follow-up period, the Visian ICL vault remained appropriate in each eye, and bilateral UDVA remained 20/30- OU. That being said, it was encouraging to see this patient’s BCVA improve after ICL implantation, which can be attributed to less minification with intraocular correction compared to spectacles. The optics of a high-powered external myopic lens can minify observed objects.
Prior to ICL implantation, the patient was being monitored by a retina specialist for risk of choroidal neovascularization associated with myopic degeneration. After undergoing ICL surgery, she received one anti-VEGF injection for choroidal neovascularization in her left eye. Visual acuity remains stable in both eyes, and she has not received additional injections since that time.
1. Fernandez-Vega-Cueto L, Lisa C, Esteve-Taboada JJ, et al. Implantable collamer lens with central hole: 3-year follow-up. Clin Ophthalmol. 2018;12:2015-2029.
2. Gao J, Liao RF. Correlation between white-to-white diameter and ciliary sulcus diameter of high myopia eyes [in Chinese]. Zhonghua Yan Ke Za Zhi. 2013;49(7):627-632.
3. Dougherty PJ, Rivera RP, Schneider D, et al. Improving accuracy of phakic intraocular lens sizing using high-frequency ultrasound biomicroscopy. J Cataract Refract Surg. 2011;37(1):13-18.
4. Oh J, Shin HH, Kim JH, et al. Direct measurement of the ciliary sulcus diameter by 35-megahertz ultrasound biomicroscopy. Ophthalmology. 2007;114(9):1685-1688.
5. Lee DH, Choi SH, Chung ES, Chung TY. Correlation between preoperative biometry and posterior chamber phakic Visian Implantable Collamer Lens vaulting. Ophthalmology. 2012;119(2):272-277.
6. Reinstein DZ, Archer TJ, Silverman RH, et al. Correlation of anterior chamber angle and ciliary sulcus diameters with white-to-white corneal diameter in high myopes using Artemis VHF digital ultrasound. J Refract Surg. 2009;25:185-194.
7. Reinstein DZ, Lovisolo CF, Archer TJ, Gobbe M. Comparison of postoperative vault height predictability using white-to-white or sulcus diameter-based sizing for the Visian implantable collamer lens. J Refract Surg. 2013;29:30-35.
8. Price MO, Price DA, Bucci FA Jr, et al. Three-year longitudinal survey comparing visual satisfaction with LASIK and contact lenses. Ophthalmology. 2016;123(8):1659-1666.