
In February, I conducted a survey of 47 attendees of the 2021 Cedars Aspens annual meeting to gain insight into physician preferences on learning about and prescribing new drug products. This report summarizes the survey results. (For other surgeons' thoughts on this topic, see the sidebar Say Anything, at the end of this article.)
SURVEY Respondents
Age and sex. Most survey respondents fell into one of two age groups: 45 to 54 years (44.7%) or 55 to 64 years (38.3%). Of all respondents, 52.2% were men and 47.8% were women.
Practice setting. The survey respondents practice in a variety of settings, including 42.6% in a private ophthalmology and optometry group setting, 21.3% in an academic setting, 19.1% in a small private practice with fewer than three ophthalmologists, and 14.9% in a multispecialty group practice. The remaining respondents reported working as employed physicians. Additionally, about half (52.2%) of the respondents reported that they work with residents or fellows.
Years in practice. The majority of survey respondents (51.1%) reported having been in practice for more than 20 years, 31.9% reported having been in practice for 10 to 20 years, and the remaining reported working for either 5 to 10 years or less than 5 years.
DRUG REPRESENTATIVE PRACTICE VISITS
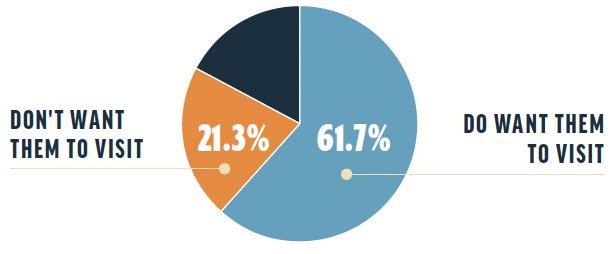
Overall interest. Of the survey respondents, 61.7% reported that they want drug representatives to visit their practices; 21.3% reported that they do not want drug representatives to visit. The remaining respondents noted that their interest in this activity hinged on certain conditions, including the safety of the visits during the COVID-19 pandemic, the usefulness and frequency of the visits, and whether samples would be provided during the visit.
Before and during the COVID-19 pandemic. Drug representatives were allowed in 91.5% of survey respondents’ practices before the onset of the pandemic. Now, during the pandemic, 48.9% of respondents are allowing drug representatives in their offices, and 38.3% of respondents are not. The remaining respondents reported that drug representatives are allowed in their offices only by appointment, on a limited basis, with proper COVID-19 screening, only to drop off samples, or to meet for lunch in the practice conference room.
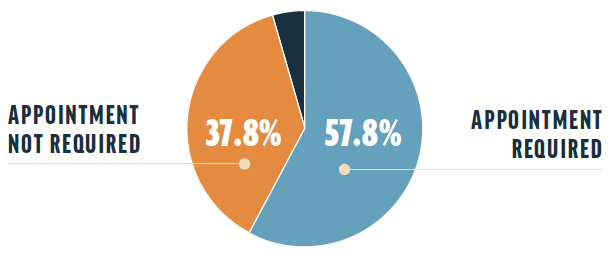
Need for appointments. A total of 57.8% of respondents reported that an appointment is required for a drug representative to visit their practices, whereas 37.8% reported that an appointment is not required. Others reported that drug representative visits are entirely prohibited at this time or that interactions with drug representatives are fully virtual during the pandemic and were by appointment only before the pandemic.
Points of contact. A variety of communication practices were reported to be preferred for drug representative visits. A total of 44.7% of respondents prefer for representatives to talk with the ophthalmologist(s), 34.2% prefer for representatives to talk with anyone but the ophthalmologist(s), and 7.9% prefer for representatives to engage with only the technician(s). The remaining respondents prefer for representatives to speak with only the administrator(s) or only the optometrist(s).
Physician-representative interactions. When survey respondents were asked whether drug representatives speak to them during their visits, 75.6% said yes and 8.9% said no. The remaining respondents reported that drug representatives speak to them when they are available, speak to them only when the representative provides a meal for the entire practice, sometimes speak to them, speak to them depending on certain variables, or speak to them or their prescribing optometrist.
Technician-representative interactions. When asked whether drug representatives speak to the survey respondents’ technicians during practice visits, 82.2% said yes, 15.6% said no, and the remaining respondents said sometimes.
Preference for lunch. When asked whether they prefer drug representatives to bring lunch when they visit, 48.9% of respondents said yes and 34% said no. The remaining respondents reported that they no longer prefer for lunch to be provided due to the pandemic, that a snack or coffee is appreciated, or that it does not matter.
Preferred time of drug representative visits. Of the survey respondents, 38.3% prefer drug representatives to visit by appointment, 29.8% prefer lunchtime visits, 10.6% prefer walk-in visits, 8.5% prefer morning visits, 8.5% prefer no visits, and the remaining respondents prefer afternoon visits.
PRIOR AUTHORIZATION AND NEW DRUG PRESCRIBING PREFERENCES
Most survey respondents (95.7%) reported that their offices do prior authorizations (PAs) for patient prescriptions. The remaining respondents reported that they do only when needed or do as few as possible. A technician does the PAs in 57.4% of respondents’ practices, administrative staff members do them in 36.2% of respondents’ practices, and the remaining respondents reported that the ophthalmologist does them or that they are uncertain who does them.
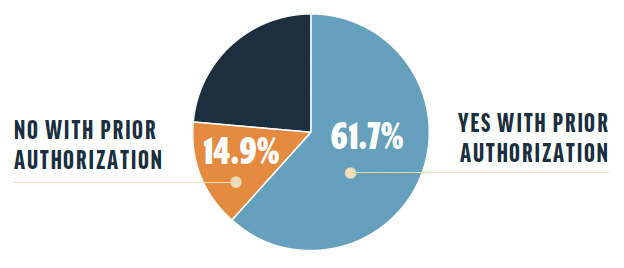
When asked whether they would prescribe a new drug if a PA were required, 61.7% of survey respondents responded yes and 14.9% responded no. The remaining respondents answered that it depends on the need, their staff handles this task, they would rather use an alternative drug option that does not require PA, it depends on how time-consuming the PA process is, or it is becoming less likely.
DRUG SAMPLE PREFERENCES
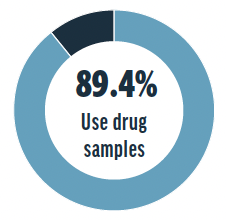
Drug samples are used in 89.4% of survey respondents’ practices, but they are not allowed in 8.5% of respondents’ practices. The remaining respondents reported that drug samples are not used in their practices. For obtaining new drug samples, survey respondents’ most preferred method was delivery by drug representative, the second most preferred method was by online order, and the least preferred method was by mail.
Once comfortable with a new drug, 39.1% of respondents reported that their preferred delivery method for receiving samples is via drop-off by the drug representative, with the ophthalmologist providing their signature on a tablet. A total of 19.6% of respondents stated that they are too busy and simply want the samples to be available when needed, 19.6% stated that their preference is for the drug representative to restock the sample cabinet as needed, 13% reported a preference for online ordering, and 8.7% reported a preference for mail.
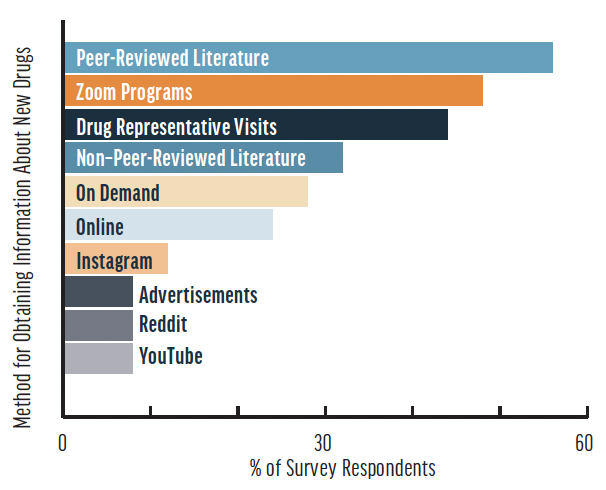
The most commonly reported method for how survey respondents’ residents or fellows obtain information about new drugs was by peer-reviewed literature (56%), followed by Zoom programs (48%), drug representative visits (44%), non–peer-reviewed literature (32%), on demand (28%), online (24%), Instagram (12%), advertisements (8%), Reddit (8%), and YouTube (8%). Eight of the survey respondents (32%) reported that their residents or fellows use the same methods they use.
Survey respondents’ reported that patients in their practices receive education on new drug administration from an ophthalmologist (73.3%), a technician (60%), and/or an optometrist (40%). One survey participant reported that, in their practice, the ophthalmologist, optometrist, and technician all instruct patients on how to administer drugs with new routes of delivery. One respondent noted that the ophthalmologist, optometrist, technician, and scribe all participate in this effort, and another responded that the drug company should provide an educational video demonstrating proper administration.
1. Have you incorporated a new pharmaceutical treatment into your practice as a direct result of a visit from a drug representative?
2. How willing would you be to incorporate into your practice a new pharmaceutical treatment that requires a prior authorization (PA) from insurance companies?

Robert F. Melendez, MD, MBA
Juliette Eye Institute, Albuquerque, New Mexico
1. “I currently use OSRX Omni compounded formulations for all of my refractive and cataract surgery patients. This was a direct result of a drug representative's sharing this information with my practice. I’ve been using these formulations since December 2020. The Omni drop I use for refractive surgery is a combination formulation of prednisolone phosphate 1% and gatifloxacin 0.5%. The Omni drop I use for cataract surgery includes prednisolone phosphate 1%, moxifloxacin 0.5%, and bromfenac 0.075%. These compounded formulations are less expensive than an individual prescription for each drop would be. The drops have been efficacious in preventing endophthalmitis and prolonged inflammation following surgery.”
2. “I am less willing to incorporate a drug that requires PA into my practice. The additional time required to complete PA can delay the delivery of care and decrease efficiency in the clinic. Therefore, if a drug requires PA, I am less likely to prescribe it unless the patient absolutely needs it. However, if the drug has shown greater efficacy than other options that do not require PA, it is worth the extra time.”

Amenze Osa Oriaifo, MD
Cataract, glaucoma, and comprehensive eye specialist and surgeon, Central Texas Eye Center, San Marcos, Texas
1. “We have so many treatment options in ophthalmology. Sometimes, a representative stopping in or sending an email reminds us of the tools available in our armamentarium. If it is appropriate and potentially beneficial to a patient, I will offer it. From drops to implantable drug therapy, a representative’s time has occasionally made a difference in my practice.”
2. “I am willing to and have done so if I feel my patients will benefit from the treatment. Whatever companies can do to make this easier on billing departments will benefit all parties involved. Ideally, in the future, and if needed, the physician and their team can provide a diagnosis code and a QR code. The patient could follow the QR code link to input their remaining insurance information to run PAs, and the information could then be emailed to the clinic. This format could potentially streamline the approval process and get patients the care they need faster.”

Dagny Zhu, MD
Medical Director and Partner, Nvision Eye Centers, Rowland Heights, California
1. “Pharmaceutical treatments for dry eye and ocular surface disease continue to advance rapidly, and drug representatives are often one of the first ways I learn about these new medications. I am a cornea, cataract, and refractive surgeon, and my patients are eager to have surgery. Many require optimization of their corneal surface before (and sometimes after) surgery, so I am always open to trying new drops in hopes of finding something that works faster and better for my dry eye patients.”
2. “It depends on the product. If I found the treatment to be clinically superior to all the other drugs within its class, I would certainly utilize any means necessary to help my patients gain access to that medication. However, if we found that the new treatment was no better than other medications, I would not bother with the hassle of PA. This is especially true in more urgent cases where I need the patient to start the appropriate therapy as soon as possible. A PA can, unfortunately, delay care and put the patient’s eye health at risk, so I prefer to prescribe the best medication they can actually get their hands on.”

Joseph Tauber, MD
Anterior segment surgeon, Tauber Eye Center, Kansas City, Missouri
1. “I have been pleasantly surprised with the safety and efficacy of oxymetazoline hydrochloride ophthalmic solution 0.1% (Upneeq, RVL Pharmaceuticals), which was first introduced to me during a drug representative visit. I had underestimated the number of patients who want treatment for ptosis, and patient satisfaction, including that of my own family members, has been high with this drug. This experience will increase my openness to learning from drug representatives.”
2. “My office staff and I are willing, though certainly not eager, to work through PAs for medications with efficacy in areas that do not have less onerous ways to treat patients effectively.”




