Can small-incision lenticule extraction (SMILE) really be more than a decade old already? Admittedly, the investigational procedure described by Walter Sekundo, MD, in CRST Europe in April 2007 (see the accompanying excerpt below) was not exactly SMILE. It still involved the creation of a stromal flap, rather than a pocket. But it shared with SMILE the paradigm-shifting difference of eliminating the excimer laser, making it a one-step refractive surgical procedure. For the first time, an all-laser procedure could be performed with only the femtosecond laser, rather than requiring a midprocedure shift of the patient between lasers.
Femtosecond Lenticular Extraction
This investigational new procedure is unique to the VisuMax laser and currently performed by only two clinical investigators in Germany.
By Walter Sekundo, MD
I am a clinical investigator of a new technique called femtosecond lenticular extraction (FLEx). This new innovation in corneal surgery surgery uses a femtosecond laser alone, compared with other procedures that use an excimer plus femtosecond laser.
Marcus Blum, MD, of Erfurt, Germany, and I introduced FLEx at the 2006 American Academy of Ophthalmology Annual Meeting, in Las Vegas. Although the procedure is not yet approved, FLEx has demonstrated the enormous potential of VisuMax (Carl Zeiss Meditec), because the quality of cut allows us to extrude the refractive lenticule from the cornea. The following information provides an account of how the procedure works.
FLEx is not LASIK; the healing responses of the two procedures are quite different. For instance, there is no initial overcorrection as seen with the excimer laser. The cornea is not ablated during FLEx like it is when an excimer laser is used. Therefore, energy is not lost at the periphery of the ablation, and prolate refractive zones are similar to wavefront-optimized excimer laser ablation results. Our results with FLEx, which is a single-step procedure (Figure), are much better than we ever expected.
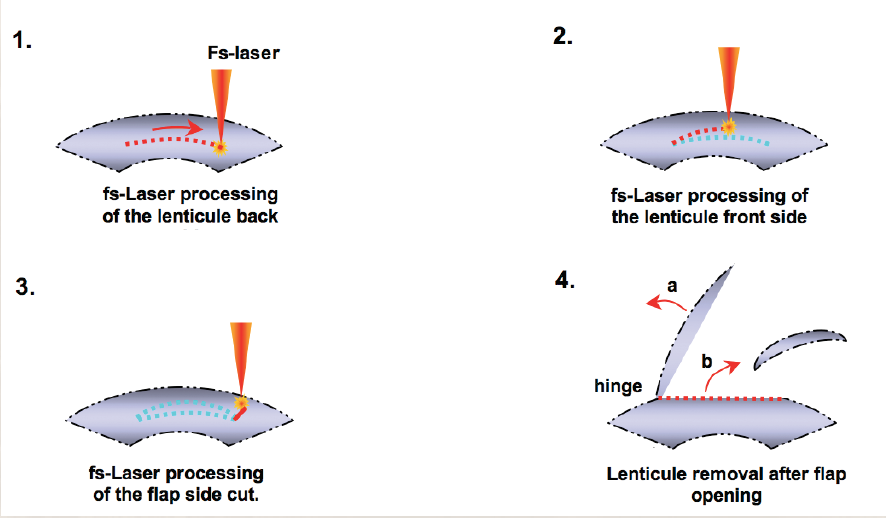
Figure. FLEx is a single-step procedure.
HOW IT WORKS
During FLEx, the femtosecond laser creates a refractive and a nonrefractive cut in a single step. After the flap is lifted, a piece of stromal corneal tissue (ie, refractive lenticule) is removed. Next, the flap is repositioned in the usual fashion. This new procedure has an enormous potential. Currently, we have 3-month results; however, Carl Zeiss Meditec will soon release the 6-month follow-up data. At 3 months, all patients treated with FLEx were 20/40 or better uncorrected. If we can customize and further improve upon the FLEx procedure, surgeons could conduct an entire lamellar refractive procedure with the femtosecond laser alone.
MY EXPERIENCE
The final version of the VisuMax femtosecond laser was released for clinical validation in February. We have treated 32 myopic eyes using the VisuMax/MEL-80 platform (Carl Zeiss Meditec). The results have been spectacular; a few patients have experienced 20/10 uncorrected vision 1 day following surgery. I have cut very thin flaps as well as thick flaps (range, 100–150 µm). In the more than 50 procedures I have performed so far, I have never created a buttonhole, tear, or any other complication that one experiences with normal microkeratomes.
We expect a further intense phase of clinical research before this procedure might become commercially available; however, FLEx has the potential to revolutionize the entire course of corneal refractive surgery. This is the fascinating procedure of the future.
Dr. Sekundo called his procedure femtosecond lenticular extraction (FLEx), and he noted that it was being performed by only two investigators.1 When he and his colleague Martin Blum, MD, had introduced FLEx the previous year at the AAO Annual Meeting, it was a striking innovation. The femtosecond laser was already beginning to replace mechanical microkeratomes for flap creation in LASIK, but that procedure required use of the excimer laser for stromal ablation. Protocols for moving patients from one laser to the other could be cumbersome. FLEx offered the possibility of performing the complete refractive surgical procedure in one place, with one laser—the VisuMax femtosecond laser (Carl Zeiss Meditec).
At that time, in 2007, only a few FLEx procedures had been performed, and 6-month follow-up data had not been released. When I wrote about the all-in-one procedure for CRST in 2010, it was still in its early stages, but by that time the name SMILE had been coined.2 No SMILE procedures had yet been performed in the United States, but investigation and development of the technique was taking place at several international sites.
In that 2010 article, I described two procedures, collectively referred to as refractive lenticule extraction (ReLEx). The first was ReLEx FLEx, in which a stromal flap and a refractive lenticule were created with the femtosecond laser, and the second was ReLEx SMILE, in which the lenticule was removed through a small incision. In the latter procedure, no flap creation and repositioning were required.
At the time, I said this about SMILE: “The advantages of the SMILE concept are that it is less invasive, there is no chance for flap dislocation, the cornea inherently maintains a greater biomechanical structure, and there should theoretically be a shorter healing cycle.” It remains true today, except that, with more than 1 million SMILE procedures now performed, we can now skip the word theoretically.
SMILE, still performed with the VisuMax femtosecond laser, received FDA approval for the reduction of myopia from -1.00 to -8.00 D, with 0.50 D of cylinder or less, in 2016. That approval was based on the results of a pivotal study including 336 eyes treated at five US investigational sites.3,4
NEW INDICATIONS
In October, the FDA expanded the treatment indications for SMILE with the VisuMax femtosecond laser. With this approval, SMILE can now be used to treat sphere from -1.00 to -10.00 D and cylinder up to 3.00 D, with a manifest refraction spherical equivalent (MRSE) up to 11.00 D (according to the FDA treatment notification, between -10.01 and -11.00 D). The SMILE cap incision size can now be reduced to 60° if desired, compared with 90° for the initial myopia-only indications.
I believe this will enable us to use SMILE to treat 85% to 90% of our refractive surgery patients. Benefits include smaller incisions, potentially greater biomechanical stability, and reduced impact on corneal nerves, which may lead to less postoperative dry eye.5-7
These new indications were based on the results of a multicenter clinical trial, in which spherocylindrical SMILE was performed in 307 eyes and sphere-only SMILE in 50 eyes.8 Investigators treated -1.00 to -10.00 D sphere and cylinder of up to 3.00 D. In eyes with astigmatism of -0.50 D or less, only sphere was treated. Significant amounts of spherical myopia (mean MRSE, -5.93 D) and astigmatic myopia (mean MRSE, -5.41 D, cylinder -1.52 D) were treated.
CLINICAL OUTCOMES
Patients were followed for 1 year, and no enhancements were performed. In this high myopic spherical equivalent group, 84% of eyes had 20/20 or better postoperative UCVA and 99% had 20/40 or better UCVA at 6 months (Figure 1).
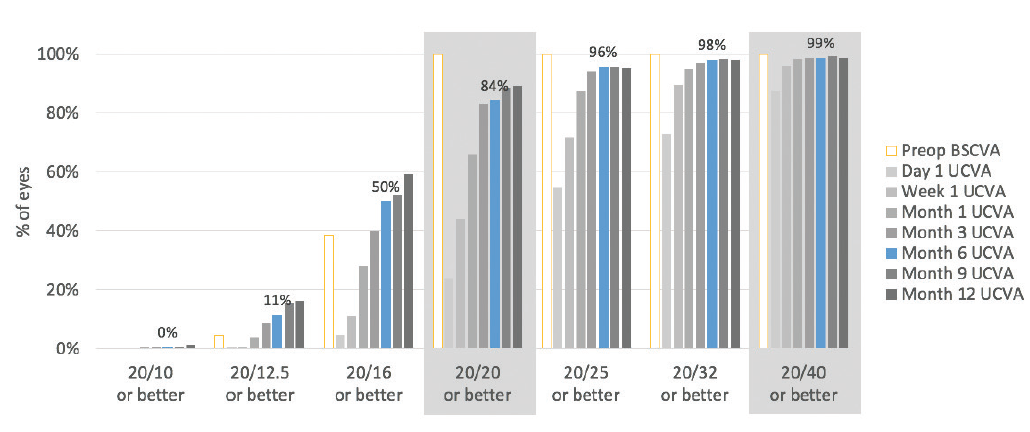
Figure 1. Six months after surgery, 99% of eyes had UCVA of 20/40 or better and 84% had UCVA of 20/20 or better.
When these results are compared with the most recent FDA trials of LASIK technologies of other manufacturers—Contoura Vision (Alcon), iDesign (Johnson & Johnson Vision), and EC-5000 (Nidek)—the SMILE 6-month results are essentially equivalent (Figure 2).8-11 In the Contoura Vision results, 89% of eyes had 20/20 UCVA or better; with iDesign, 83%; and with the EC-5000, 89%.
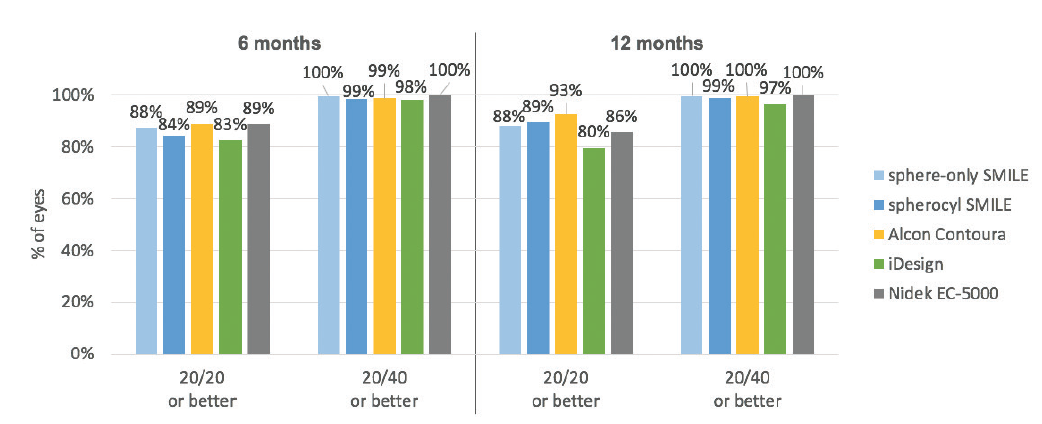
Figure 2. UCVA results after SMILE are comparable to results after LASIK.
Regarding the accuracy of targeted MRSE correction, SMILE results again were comparable to those of recent FDA data on LASIK technologies (Figure 3).8-11 In spherocylindrical SMILE eyes, 94% were within ±0.50 D of the target refraction at 6 months postoperative. That degree of accuracy was achieved in 93% of Contoura Vision eyes, 69% of iDesign eyes, and 91% of EC-5000 eyes.
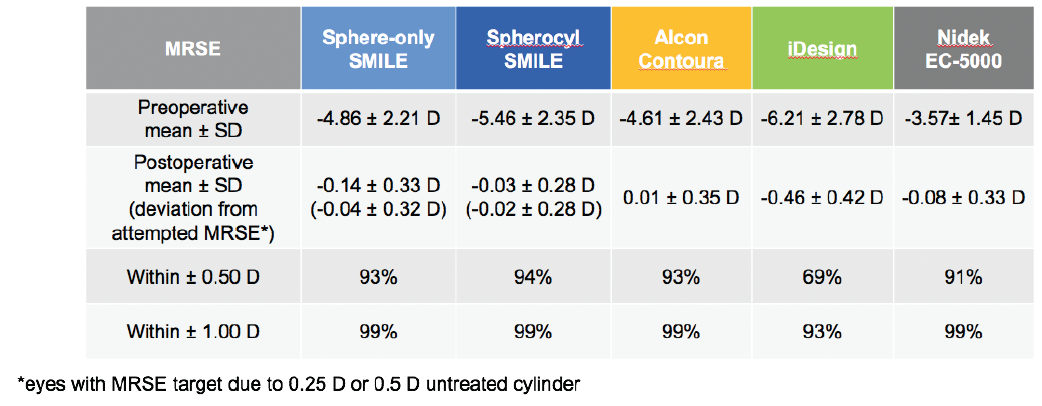
Figure 3. Predictability and accuracy of MRSE correction are comparable to or better than LASIK at 6 months after surgery.
SMILE also compared well to the other devices in the accuracy of cylindrical correction within ±0.50 D of target at 6 months postoperative: SMILE, 88%; Contoura Vision, 90%; iDesign, 85%; and EC-5000, 91% (Figure 4).8-11 It is worth noting that larger amounts of astigmatism and significantly higher spherical equivalent values were treated in the SMILE and iDesign trials than in the Contoura Vision and EC-5000 trials.
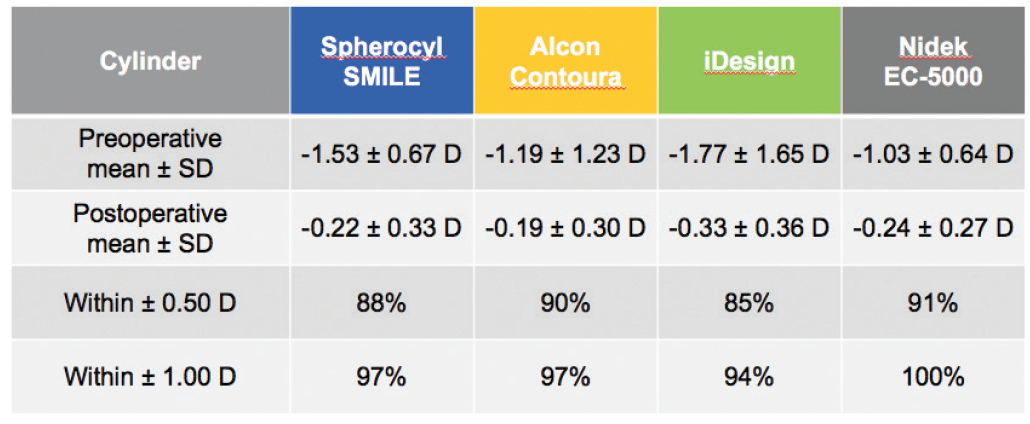
Figure 4. Predictability and accuracy of cylinder correction 6 months after surgery are comparable to or better than LASIK results.
POTENTIAL IMPACT
In addition to the advantages I enumerated earlier, SMILE produces a more prolate cornea than traditional laser vision correction when the disc of tissue is removed, and this may result in relatively better nighttime vision compared with traditional excimer laser ablations. SMILE is also dose-independent because all treatments take the same amount of time. Furthermore, because the patient interface is in contact with the cornea and surgery is performed within the corneal stroma, SMILE reduces environmental concerns. There is less need to worry about temperature, barometric pressure, humidity, and particulate matter, all of which can be concerns with excimer laser procedures.
Now, with expanded treatment indications for SMILE, the benefits of this minimally invasive, flapless procedure can be offered to more refractive surgery patients. Using the straight-out-of-the-box algorithm for SMILE, without nomogram adjustments, investigators in the clinical trial achieved outcomes comparable to those achieved with excimer laser technology with the benefit of 23 years of optimization. (Editor’s note: SMILE can currently be performed only with the VisuMax femtosecond laser; however, two related procedures are being studied by Schwind eye-tech-solutions and Ziemer. For more information on those techniques, scroll down to see On the Horizon.)
On the Horizon
Schwind’s Lenticule Extraction Procedure
By Theo Seiler, MD, PhD
Schwind eye-tech-solutions has developed a corneal lenticule extraction procedure comparable with the small-incision lenticule extraction (SMILE) technique introduced by Carl Zeiss Meditec. In my opinion, however, the Schwind technique is smarter, and the learning curve is significantly shorter.
The repetition frequency of the new Schwind femtosecond laser is currently 3 MHz, but it can be tuned up to 10 MHz. The cutting pattern is more intelligent because it does not need the cylindrical cut at the edges as used in SMILE. In consequence, this leads to less healing response. The curvature of the Schwind’s patient interface is stronger compared with the VisuMax femtosecond laser (Carl Zeiss Meditec), resulting in fewer intrastromal surface irregularities. Cyclotorsion control and eye-tracking technology will be utilized in the Schwind lenticule extraction procedure.
The proposed Schwind lenticule extraction procedure will use two small incisions, 90° away from each other, one incision leading to the anterior surface and the other to the posterior surface of the lenticule. This will make the procedure easier and the learning curve shorter.
In India, we performed the procedure for the first time in a series of patients (N = 20) in order to establish nomograms and improve parameters. In the first group of patients, we had significant undercorrection, but in a second group the undercorrection rate was as low as 5%. When I did these procedures in India, my personal impression was that the procedure is easier to perform compared with the SMILE procedure.
During brainstorming seminars, we are already planning customized treatments based on topography or wavefront analysis. With the built-in cyclotorsion and eye-tracking technologies, the results of correction of myopic astigmatism will be significantly improved, comparable to the improvement that cyclotorsion and eye tracking brought to LASIK 15 years ago. (It is high time that these technologies were brought to SMILE.)
To the best of my knowledge, Schwind eye-tech-solutions is expecting to apply for the CE Mark for the device in spring 2019, and, therefore, I assume that it will be available on the market at earliest by the second quarter of 2019.
Ziemer’s Lenticule Extraction Procedure
By Christian Rathjen, PhD
Still in the developmental phase and in response to trends in today’s corneal refractive surgery market, Ziemer is working on a lenticule extraction procedure that will be performed with the company’s Femto LDV Z8 femtosecond laser. This procedure will apply the same low-energy principle used by the laser during LASIK and other corneal procedures, but it will incorporate a new scanning algorithm to execute the lenticule extraction procedure.
Before I share preliminary information on this procedure, I want to make it clear that, as a company, Ziemer strongly believes that LASIK is alive and well. This new lenticule extraction procedure is not meant to replace or be an upgrade to LASIK or even PRK, but rather an alternative that surgeons can choose to perform when appropriately indicated in their patients. We are still very much in favor of modern LASIK because of its outstanding safety and performance; however, in certain markets, lenticule extraction seems to be a valuable supplement to the refractive surgeon’s portfolio.
With that said, we are excited by the potential of Ziemer’s new lenticule extraction procedure. For the past 6 months, we have been working with three surgeons to gather early hands-on experience. The feedback we have received regarding the overall quality and precision of the scanning algorithm and the lenticule extraction procedure is encouraging enough to continue our development. To date, surgeons have seen the same cut quality in these lenticule procedures as in LASIK femtosecond flaps performed with the Femto LDV.
We expect to present data on the procedure at meetings in the coming year, and we plan to make the first software module available for experienced corneal surgeons. Initially, the refractive indication will be for the treatment of myopia.
1. Sekundo W. Femtosecond lenticular extraction. CRST Europe. April 2007.
2. Doane JF. VisuMax femtosecond laser offers an all-in-one refractive procedure. CRST. March 2010.
3. Zeiss receives US FDA approval for VisuMax SMILE vision correction procedure, the latest advancement in laser eye surgery [press release]. Carl Zeiss Meditec. September 14, 2016. https://www.zeiss.com/meditec/int/media-news/press-releases/us-fda-approval-visumax-smile-vision-correction-procedure-in-laser-eye-surgery.html. Accessed October 22, 2018.
4. VisuMax femtosecond laser. Summary of safety and effectiveness data (SSED). https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150040B.pdf. Accessed October 25, 2018.
5. Denoyer A, Landman E, Trinh L, Faure JF, Auclin F, Baudouin C. Dry eye disease after refractive surgery: comparative outcomes of small incision lenticule extraction versus LASIK. Ophthalmology. 2015;122(4):669-676.
6. Spiru B, Kling S, Hafezi F, Sekundo W. Biomechanical properties of human cornea tested by two-dimensional extensiometry ex vivo in fellow eyes: femtosecond laser-assisted LASIK versus SMILE. J Refract Surg. 2018;34:419-423.
7. Sinha Roy A, Dupps WJ Jr, Roberts CJ. Comparison of biomechanical effects of small-incision lenticule extraction and laser in situ keratomileusis: finite element analysis. J Cataract Refract Surg. 2014;40:971-980.
8. Carl Zeiss Meditec. Data on file.
9. STAR S4 IR Excimer Laser System iDesign Advanced WaveScan Studio System. Summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf/P930016S044B.pdf.
10. Allegretto Wave Eye-Q Excimer Laser. Summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020050S012B.pdf.
11. Nidek EC-5000 Excimer Laser System. Summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf/P970053S011b.pdf.