Small-incision lenticule extraction (SMILE) was introduced 7 years ago, and last year Carl Zeiss Meditec announced that 1 million eyes have been treated to date.1 However, the patient’s ocular journey does not end after laser refractive surgery. Just like patients who have undergone LASIK or PRK, patients who undergo SMILE may need cataract surgery as they age. As we see an increasing number of post-SMILE patients presenting for cataract surgery, we will have to answer this question: Should these patients be treated differently from those who have undergone other types of corneal laser refractive surgery?
Evidence to date suggests there is no difference for a surgeon operating on an eye that has undergone SMILE compared with any other corneal laser refractive surgery, such as LASIK or PRK. Most surgeons have at least a basic understanding of which IOL calculation formulas are the most accurate for patients after corneal refractive surgery, given sufficient patient data. Surgeons do not need to change their pre- or postoperative care routines for a SMILE patient, and the surgical course itself will also be no different from any other eye.
IOL POWER SELECTION
Just as with any other corneal laser refractive surgery, the most interesting points of discussion surrounding cataract/IOL surgery after SMILE are how to select the correct lens to implant and how to improve IOL calculations in postrefractive surgery eyes.
I strongly believe that, with a few exceptions, patients who had a corneal refractive procedure should receive monofocal IOLs. Even in virgin eyes, a monofocal IOL provides a superior quality of vision—based solely on the optical principles involved—compared with a multifocal IOL. Should the patient want to decrease dependence on glasses after IOL surgery, this can effectively be achieved with the use of monovision techniques. If refracted and titrated properly, most of my patients are able to accept some range of monovision correction. Furthermore, the great advantage of using monofocal IOLs is that lenses with lower rates of posterior capsular opacification (PCO) can be chosen. Additionally, we can perform corneal laser enhancements over time if corneal astigmatism changes with age.
Given these benefits of monofocal IOLs, I can avoid having to worry about the uncontrolled risks that come with implanting a multifocal IOL, such as poor subjective quality of vision, reduced contrast sensitivity, low tolerance to even small degrees of ametropia, and the possibility of poor neural adaptation. These complications, if severe enough, could lead to the patient needing a high-risk lens exchange procedure.
DISCUSSION
In the case presented in the accompanying sidebar, my colleagues and I looked at the patient’s preoperative corneal wavefront measurement to determine what type of lens would be most appropriate. As is the case with most refractive surgery patients, there was an increased amount of corneal spherical aberration due to the original myopic SMILE treatment (Figure). The amount we measured was in a zone where it would contribute to increasing the eye’s depth of focus, but it was not high enough to be considered toxic to the visual system. Therefore, we decided to implant a monofocal lens with a slight degree of asphericity.
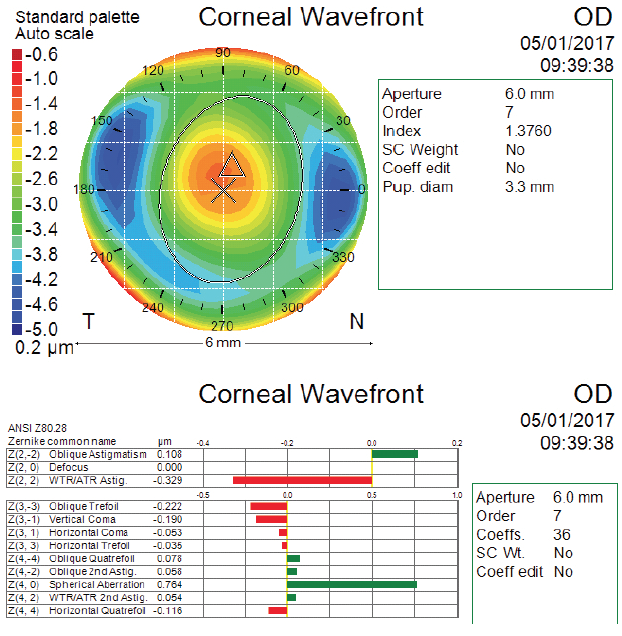
Figure. Atlas (Carl Zeiss Meditec) corneal wavefront scan after SMILE and before cataract surgery for the right eye.
As noted previously, IOL power calculation for SMILE eyes should be done in the same manner and using the same formulas as for post-LASIK eyes. The amount of spherical aberration in the cornea will influence the calculation. Once a large quantity of post-SMILE cataract surgery data has been gathered, there may be small differences, but we are currently seeing reports of outcomes similar to those that can be expected in post-LASIK eyes.
CASE STUDY
Background: SMILE Surgery
- 63-year-old woman underwent SMILE at the London Vision Clinic in 2012
- Preoperative refractions: -7.50 -1.75 x 98° (20/20-2) OD / -10.25 -0.75 x 73° (20/20-2) OS
- Intended outcomes: -1.75 D OD / plano OS
- Refractions 3 years postoperative: -2.00 D (20/20-2) OD / 0.00 -0.25 x 152° (20/20-1) OS
Patient Complaints
- Patient returned January 2018 complaining of blurred vision in both eyes and difficulty with activities in low light conditions
- Uncorrected distance visual acuity (UDVA): 20/80+1 OD / 20/40-2 OS
- Manifest refractions: -2.25 -1.50 x 90° (20/40+2) OD / +1.00 -2.00 x 89° (20/32) OS
- Slit-lamp examination: grade 3 nuclear sclerosis with numerous central lens vacuoles OD / grade 2 nuclear sclerosis with a few vacuoles and peripheral cortical spokes OS
- Keratometry (K): 38.07 and 37.75 @ 81º OD / 36.07 and 35.22 @ 177º OS
- Contrast sensitivity: Significantly below the normal range at 3, 6, 12, and 18 cycles per degree OU
Surgical Course
- Preparation for intraocular surgery and IOL power calculation were performed in the same manner as for a patient who had undergone myopic LASIK or PRK
- The IOL power was chosen based on results from the Barrett True K, Haigis-L, and Shammas formulas
- An AcrySof SN60WF lens (Alcon) was selected, with a refractive target of -1.625 D in the patient’s right eye and plano in her left
Three-Month Postoperative Visit
- Distance UCVA: 20/50+2 OD / 20/32-2 OS
- Near UCVA: 20/20 OD / 20/80 OS
- Distance BCVA: -1.50 -0.75 x 62° (20/20+1) OD / -0.75 (20/25-2) OS
- Contrast sensitivity: Normal levels in both eyes
- Slit-lamp examination: Unremarkable, with clear and well centered lenses OU
The final piece of the puzzle for IOL calculation is noting the shifted power of the epithelium after corneal surgery and achieving a better understanding of its refractive role. The epithelial profile in SMILE is slightly different from the profile in LASIK, so this is where small changes in formulas may lie. A larger study will help determine whether any small changes must be made to existing formulas.
1. Zeiss celebrates 1 million SMILE laser vision correction procedures [press release]. Carl Zeiss Meditec. Jena, Germany. September 28, 2017.