My Go-To Techniques for a Rock-Hard Nucleus
Lisa Brothers Arbisser, MD

My technique for large brunescent cataracts respects the critical advantages of a standard, 5-mm continuous curvilinear capsulorhexis (CCC), including the safety net of optic capture and the long-term advantages of optic edge coverage. The purpose of hydrodissection is to free lens contents from the capsular bag to create a mobile nucleus without placing stress on the zonules. Freeing the nucleus is essential for most phaco disassembly techniques as well as for clean cortical removal, which reduces postoperative inflammation and fibrosis. The maneuver is often taught as simple irrigation with a cannula through the clear corneal incision. Emphasis is appropriately on the tip’s placement under the anterior capsular rim, and all ophthalmologists are taught to watch the fluid wave cross the midline of the nucleus and exit around it. I would argue, however, that hydrodissection should be understood as a more complexly nuanced maneuver, not just simple irrigation.
HYDRODISSECTION
The technique. The proper technique for hydrodissection involves a subtle ballottement of the nucleus in concert with the expulsion of irrigation fluid from the syringe. This is effected by a slightly downward movement of the tip of the cannula (once it is seated in the correct plane) to push the nucleus somewhat posteriorly, thereby burping the capsular bag and encouraging the fluid to dissect around the equator. Downward movement is alternated with an equally subtle lifting of the cannula to apply slight downward pressure on the posterior lip of the clear corneal incision, thereby burping the anterior chamber (AC).
This seesaw-like action pushes the nucleus away from the rim of the anterior capsule, eliminating tamponade and a buildup of pressure from the force of irrigation fluid trapped within the bag, which can lead to capsular block syndrome and posterior capsular rupture in a split second (pun intended). At the same time, the maneuver relieves pressure in the AC by slightly opening the internal valve of the clear corneal incision to allow the egress of irrigated fluid, which, if explosively relieved, can cause iris prolapse.
My preferences. I prefer to use a Mackool 23-gauge cannula on a 5-mL syringe filled with balanced salt solution (Alcon). The flattened irrigating port of this cannula creates a broad stream of fluid and is easy to place in the desired plane. All solutions are irrigated through micropore filters in my OR. My goal is to free the nucleus and epinucleus separately when the cataract is immature and together when the cataract is dense and there is no endonuclear plane that can be hydrodissected.
I believe I achieve a cleaner bag with separate irrigation and aspiration of the cortex than with the classic cortical-cleaving hydrodissection technique first described by I. Howard Fine, MD.1 Although cortical cleaving (ie, placing the cannula port directly under the rim of the anterior capsule) with the phaco tip may seem efficient, it often leaves behind thin wisps of cortex that cannot adequately occlude even the 0.3-mm opening in the I/A port to build vacuum (in a phaco machine with a peristaltic pump) for efficient removal.
These sticky wisps must be forcefully irrigated off the capsule or manually removed with a 26-gauge cannula, a process that is more time-consuming and risky in my hands than a clean removal of more substantial amounts of residual cortex with the I/A handpiece after phacoemulsification is complete. Gaining purchase on the anterior edge of the cortex facilitates clean stripping. Engaging the posterior edge of a cortical remnant often leaves behind wisps.
Capsular blowout. A capsular blowout is a devastating complication that often leads to a loss of nuclear material into the posterior segment. Because a blown capsule occurs so early in the case, a tear is vulnerable to extension when the eye is pressurized by the insertion of the phaco tip, and it cannot be visualized easily until after nuclear disassembly begins. A capsular blowout is most likely to happen when the nucleus is large and dense, such as with a brunescent cataract and in a hyperopic eye with crowded chambers. Iris prolapse, particularly in eyes with intraoperative floppy iris syndrome can cause pigment loss and atonicity of the stroma, leading to intraoperative difficulties and postoperative ocular aberrations. Both conditions are best avoided with this nuanced seesaw hydrodissection maneuver.
CHOP Technique
Problems with nuclear division and sculpting. Although laser cataract surgery and the miLoop (Carl Zeiss Meditec) can be helpful for handling brunescent cataracts, I believe that attempting to divide the entire nucleus through the posterior plate places unacceptable stress on the bag—the posterior plate of these lenses has rubbery interdigitated fibers. Also, there is a dearth of posterior cortex to protect the posterior capsule, which is often flaccid due to the large lens and concurrent zonular pathology.
I therefore never attempt to create two hemispheres with the first chop in an eye with a brunescent cataract. I never sculpt a lens, but I find that sculpting a dense lens, in particular, tends to put pressure on the subincisional zonules and requires large amounts of ultrasound energy. Instead, I rely on a technique I developed and call circumferential disassembly with cross-action vertical chop.2
The technique in detail. The first chop is made with a cross-action vertical chop on phaco burst mode setting, The goal is slowly to pry apart the two hemispheres just until the middle of the nucleus is reached to gain access to the endonucleus.
The lens is then turned slightly. Very small radial pie wedges are made—complete on the anterior but not the posterior surface of the lens. This allows the surgeon to flay the lens to engage the endonucleus within the nuclear and epinuclear framework, analogous to removing a clam from its shell. The endonucleus is not firmly adherent because of a natural division between the nucleus and epinucleus. With an immature cataract, hydrodelineation produces a golden ring. Of course, hydrodelineation is not possible with a brunescent lens, but it is possible to locate this division mechanically to debulk the nucleus in a circumferential manner while simultaneously creating the usual radial disassembly.
At this point, the nucleus should be mobile and the capsular bag well expanded by the outer shell of the nucleus, which protects the posterior capsule much like a shoe tree supports a shoe. The lens can be rotated without placing stress on the zonules. Multiple sections of endonucleus are evacuated in the safe zone within the CCC and deep in the chamber away from the endothelium, which is protected by OVDs using Arshinoff’s soft-shell technique.
Phaco burst mode works well with this technique, except during removal of the final, thin, epinuclear shell. Switching to torsional pulse mode for this step can enhance followability. With phaco burst mode, the surgeon doesn’t have to hear the occlusion bell, no matter how dense the lens, because the panel is set to a high ultrasound power commensurate with the density of the nucleus. Ultrasound (footpedal position 3) is applied only to gain purchase on the nucleus, so the instrument in the nondominant hand (a simple Rosen splitter is my preference because it has no sharp point) can be used to manually separate small pieces and assist their aspiration for removal. Because virtually all of the ultrasound energy is used while the phaco tip is occluded, ultrasound times are very low, and nearly all of the ultrasound energy is delivered within the posterior chamber.
Once the endonucleus is debulked, an additional amount of a dispersive OVD is instilled through the paracentesis, and the remaining nucleofied epinuclear shell is lifted into the AC and fed into the phaco tip with the Rosen splitter. Removal is then completed, with care taken to keep the Rosen splitter below the phaco tip to protect the posterior capsule when ultrasound bursts are applied. Snug incisions, including a 0.5- to 0.7-mm paracentesis, are important to avoid the use of excessive amounts of balanced salt solution.
Conclusion
This reliable and reproducible phaco technique is a labor of love in that it takes slightly longer than other techniques. It is also effective. One day after surgery, my technicians have had difficulty distinguishing between eyes that had the most brunescent cataracts and those that did not.
1. Fine HI. Cortical cleaving hydrodissection. J Cataract Refract Surg. 2000;26(7):943-944.
2. Arbisser LB. Phacoemulsification technique with vertical chop clam shell circumferential disassembly for brunescent cataract. Tech Ophthalmol. 2005;3(4):158-164.
OVDs for Surgery on Eyes With Dense Cataracts
Steve A. Arshinoff, MD, FRCSC

The first OVD to enter global markets was Healon (Johnson & Johnson Vision) in 1980. The introduction of OVDs enabled ophthalmic surgeons to reduce damage to the corneal endothelium during IOL implantation. The protection provided by Healon was thought to be a result primarily of its viscous-cohesive nature, which prevented IOL-corneal contact by permitting the AC to be deepened and stabilized in the presence of an open wound. Additional OVDs quickly appeared, including chondroitin sulfate 1 and 2, which were the precursors to Viscoat (Alcon), and hydroxypropyl methylcellulose.
Arguments about the relative benefits of different OVD properties began with their introduction and continue today because of the evolution of cataract surgical techniques—from intracapsular cataract extraction to extracapsular cataract extraction to phacoemulsification to laser cataract surgery—each of which changes the intraoperative environment in which OVDs are required. This article discusses phacoemulsification in 2021 and what I consider to be the optimal OVD strategy for removing a very dense cataract. I discussed the general proper use of OVDs in this space in 2019.1
Surgical requirements for a very dense cataract differ from the requirements for a moderate cataract chiefly because of the length of time that the protective OVD must remain present. The use of dispersive OVDs for surgery on eyes with dense cataracts has therefore been promoted, but this view is simplistic in my opinion.
Choose A Viscous-Cohesive OVD
The creation of the capsulorhexis requires maneuvering space and inducing pressure in front of the capsule (to prevent a tear-out). A viscous-cohesive OVD is best suited for this role, and for this reason, variants of the soft-shell technique remain preferable for dense (and other) cataracts.2 A dispersive OVD nestled against the endothelium provides prolonged retention and protection for the endothelium during long cases, and a viscous-cohesive OVD below the dispersive OVD allows pressure to be induced in front of the capsule. It is also helpful to leave a thin layer of balanced saline solution below the viscous-cohesive OVD, which may be used for a trypan blue solution in hypermature cataracts, or for a solution of xylocaine with phenylephrine or epinephrine (eg, Shugarcaine) to assist with maximal pupillary dilation.3
My preference is to use Viscoat as the dispersive OVD, Healon5 (Johnson & Johnson Vision) as the viscous-cohesive OVD, and my own xylocaine-phenylephrine formula below the OVDs as the third layer in what I term a trisoft shell technique (Figure 1).4
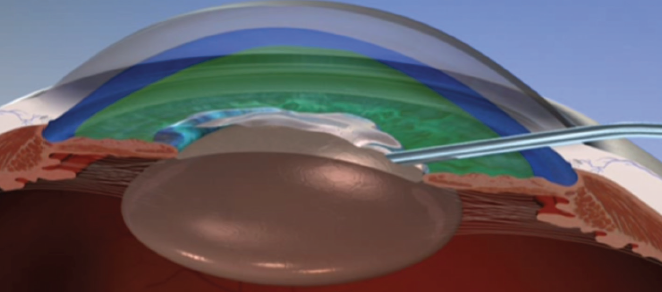
Figure 1. The layers of OVD in Dr. Arshinoff’s preferred trisoft shell technique.
Optimal Surgical Technique With OVDs
Cataract surgery is not just about OVDs, of course, so I will discuss them in the context of surgical technique. If the OVD shell comes apart and some amount of an OVD is lost, it is necessary to replace only the Healon5 because, in almost every instance, that is what has been unintentionally removed.
Capsulorhexis. After the AC has been stabilized with OVDs, the next step is to create an optimal capsulorhexis. When operating on an eye with a very dense cataract, the risk of rupturing the posterior capsule increases. Executing the first steps of surgery perfectly facilitates success with subsequent steps. The capsulorhexis should be well centered and no larger than 4.7 mm. This will allow the IOL to be easily captured in the capsulorhexis with excellent stability if the posterior capsule becomes torn. Capturing and stabilizing the IOL may be difficult or impossible with a larger capsulorhexis. The elasticity of the capsule must be taken into consideration during sizing (Figure 2).
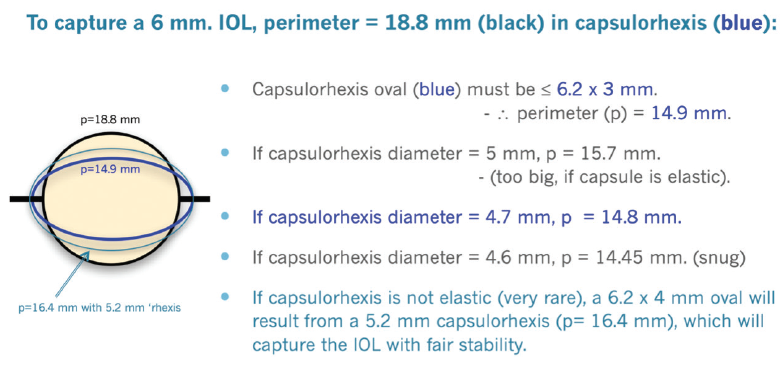
Figure 2. Dr. Arshinoff’s methodology for a proper capsulorhexis.
Hydrodissection. In dense cataracts, hydrodissection is best performed with a small-bore cannula. My preference is to use the Akahoshi II, 27-gauge beveld hydrodissection cannula (ASICO) to perform gentle subcapsular irrigation in a cortical-cleaving manner (with fluid directed upward and outward toward the anterior capsule) in all four quadrants. This approach takes a few seconds longer but leaves the OVDs undisturbed, and I find it results in a freely rotating nucleus within the capsular bag.
Phacoemulsification. When the cataract is dense, a high flow rate during phacoemulsification will wash out the OVDs. In contrast, a flow rate of 15 to 20 mL/min will disturb neither the Healon5 nor the Viscoat layer behind it, so phacoemulsification can be performed in the posterior quarter of the AC, behind the OVD layers. Resultant turbulence in the bag is equal to what would be achieved with a flow rate of 35 mL/min in the entire AC. Turbulence is the force that brings the nucleus to the phaco tip.
A phaco setting on continuously variable pulse minimizes chatter and maximizes the holding of the nucleus on the phaco tip. The nucleus is impaled just to the left of and beyond its center and held. The stabilized nucleus is chopped into six or eight segments (more pieces for dense lenses) using my favorite chopper, the Nichamin Quick Chopper (MicroSurgical Technology), and my phaco slice and separate technique.5 The bottle height is lowered to about 75 cm above the patient’s head or an IOP of 55 mm Hg on the Centurion Vision System (Alcon). (The IOP setting on the Centurion is approximately 75% of bottle height on other machines.) The purpose is to preserve the OVD structure, which protects the cornea.
With each slice of the nucleus, it becomes easier to make deeper and more complete slices into the dense nucleus. If left in situ, the retained segments help with nuclear rotation and preservation of a deep capsular bag as the entire nucleus is sliced.
IOL implantation. Once the nucleus is removed, irrigation and aspiration are easy to perform because the cortex is sparse and not sticky in dense cataracts. The second step of my ultimate soft-shell technique (IOL implantation) is to occlude the wound from the inside with a viscoadaptive OVD injected only in front of the capsular bag. The bag is then filled with balanced saline solution by injecting through the OVD. The IOL cartridge seals the wound and allows the IOL to be injected safely under the anterior capsule and into the bag while the OVD remains in front of the bag (Figure 3).
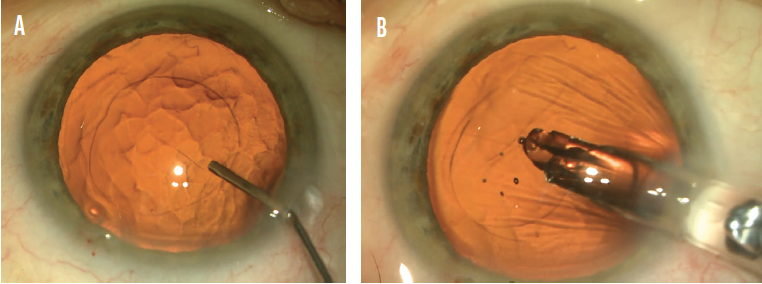
Figure 3. Dr. Arshinoff employs his ultimate soft-shell technique to implant an IOL. Surgery on an eye with a dense cataract entails injecting balanced saline solution through a viscoadaptive OVD, which has been placed only in front of the capsulorhexis, to fill the capsular bag (A). Then the IOL is injected through a cartridge, which blocks the incision, and into the capsular bag (B).
Figures 1–3 courtesy of Steve A. Arshinoff, MD, FRCSC
Conclusion
The goal with very dense nuclei is to keep the OVDs in place during phacoemulsification. The technique described herein achieves this goal and protects the cornea throughout the procedure. Nevertheless, my current favorite approach is to use a femtosecond laser to break up a very dense cataract, making phacoemulsification much easier and safer. Those cases require a different OVD strategy.6
1. Arshinoff SA. Tips for the proper use of OVDs. Cataract & Refractive Surgery Today. 2019;19(3). Accessed February 22, 2021. https://crstoday.com/articles/2019-mar/tips-for-the-proper-use-of-ovds/
2. Arshinoff SA, Norman R. Tri-soft shell technique. J Cataract Refract Surg. 2013;39:1196-1203.
3. Arshinoff SA. Ophthalmic viscosurgical devices for intumescent cataracts: pressure-equalized cataract surgery. J Cataract Refract Surg. 2015;41(7):1549-1550.
4. Arshinoff SA, Behndig A, Myers W. Intracameral medications for cataract surgery. Focal Points. American Academy of Ophthalmology. September 2018;Module 9.
5. Arshinoff SA. Phaco slice and separate. J Cataract Refract Surg. 1999;25(4):474-478.
6. Arshinoff SA. FLACS OVD press to prevent radial anterior capsular tears. Can J Ophthalmol. 2020;55:461-463.
My Preferred Chop Technique for Rock-Hard Cataracts
Maneesh Bapaye, MBBS, DNB(Ophth), FRCS(Ophth)*

Rock-hard cataracts are typically yellowish or brown in color, primarily due to the accumulation of the photo-oxidation pigment, urochrome. This type of cataract, sometimes called a catarock, is extremely dense and has a hard, brunescent nucleus. A rock-hard cataract presents a major challenge to surgeons that is often amplified by the presence of weak zonules and a thin posterior capsule. Additionally, the pupil may be small and rigid, and the endothelial cell count may be low. Careful management of each of these factors is necessary to minimize the risk of intra- and postoperative complications such as zonular dialysis, posterior capsular dehiscence, iris touch, wound burn, and delayed corneal decompensation.
Although I prefer the use of topical anesthesia in most cases, local anesthesia is indicated in certain situations such as the presence of a very small pupil, weak zonules, pseudoexfoliation, and lens subluxation and poor visibility due to a very dense arcus. If an extended surgical duration or problems with patient compliance are anticipated, I instead use peribulbar anesthesia.
Important Surgical Factors
Common techniques for surgery on eyes with a rock-hard cataract include creating a large capsulorhexis, employing a soft-shell technique to protect the endothelium, and placing pupillary expansion devices. In the absence of a good red reflex, I find it useful to stain the capsule with trypan blue dye before performing the capsulorhexis. Staining not only helps me to create the capsulorhexis, but it also improves visualization of the capsular margin, which helps to prevent inadvertent damage during phacoemulsification of the nucleus. Proper wound construction is extremely important to prevent iris prolapse, particularly if the tissue is floppy.
However, the crux of successful cataract surgery is effective management of the nucleus. It is important to minimize strain on the zonules and avoid trauma to the posterior capsule from a chopper or sharp-edged nuclear fragment. A direct chopping technique for hard cataracts is therefore preferred by most surgeons.
My Preferences
Instrumentation. I favor a long chopper, which can penetrate deep into lens matter and split the nucleus of a rock-hard cataract (Figure 4). I prefer to use a chopper with a sharp tip and inner edge, which facilitates penetration into lens material and splitting of the lens fibers. I use the Centurion phaco system, which sets the parameters for chopping hard cataracts at 50% power, a vacuum level of 450 mm Hg, and a flow rate of 28 mL/min. For the emulsification of nuclear fragments, power is usually between 65% to 90%, vacuum at 500 mm Hg, and a flow rate of 32 mL/min.
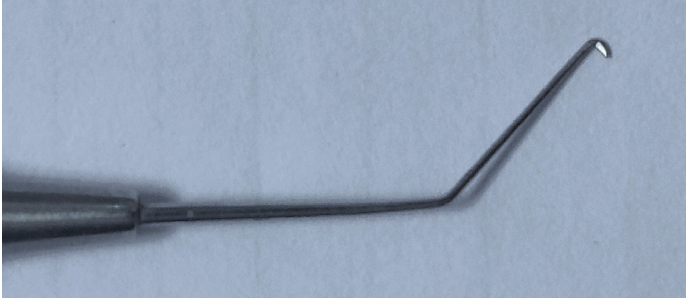
Figure 4. Dr. Bapaye uses a long chopper to penetrate deep into the lens and split the nucleus of a hard cataract.
Courtesy of Maneesh Bapaye, MBBS, DNB(Ophth), FRCS(Ophth)
Horizontal chop technique. For an eye with a hard cataract, I typically create a capsulorhexis ranging in diameter from 5.5 to 6.0 mm. This makes approaching the lens equator easier when using a horizontal chop technique—my preference. With this technique, lens material is held by the phaco probe, and the chopper is engaged as close to the lens equator as possible. After chopping in one axis, I rotate the nucleus by 180º and perform horizontal chop again before attempting a lateral split. This chop-rotate-chop maneuver dissects most of the lens fibers, and lateral separation places minimal stress on the zonules. I find this technique particularly useful in eyes with very weak zonules, as exemplified by the second case in the accompanying video.
Multilevel chop technique. I perform multilevel chop in select cases. This technique is useful for leathery, hard cataracts with a thick posterior plate. With multilevel chop, the phaco probe is engaged in progressively deeper levels of nuclear material as superficial fibers split while deeper fibers remain intact. Care must be taken not to cause posterior capsular dehiscence as the probe penetrates the nucleus. If the posterior plate is thick and difficult to crack, two instruments may be used.
After the nucleus has been divided into two hemi-nuclei, the horizontal chop technique is used to divide each hemi-nucleus into three or four pieces. A small piece of nucleus is emulsified soon after the initial chop, creating space in which to rotate other pieces and chop them. I usually lower the parameters to 50% to 60% torsional power and a vacuum level of 250 to 300 mm Hg when removing the last piece in order to avoid a sudden surge, potentially causing posterior capsular dehiscence.
Soft-shell technique. The endothelium is coated with a dispersive OVD, and a cohesive OVD is placed to flatten the anterior capsule or form the AC. Care must be taken to locate and remove every nuclear fragment, or corneal edema and inflammation may result.
Conclusion
Improvements in phaco technology have made surgery on an eye with a rock-hard cataract more routine. Nevertheless, it is prudent to have pupillary expansion devices, capsular hooks, endocapsular rings, and vitrectomy cutters accessible during these cases.
*Dr. Bapaye would like to thank Akshay G. Nair, MD, from Mumbai, India, for reviewing this article and for providing important feedback.
The Evolution of My Technique
Himani Goyal, MD

There was a time, not so long ago, when mature cataracts necessitated substantially more preparation than moderate age-related nuclear or cortical cataracts. I allotted more time for these cases—planning to use a sub-Tenon block for anesthesia instead of intracameral injection and a scleral tunnel approach instead of a clear corneal incision. The scleral tunnel was in preparation for the higher likelihood of a posterior capsular tear or possibly even a lens that was too dense to phacoemulsify, requiring expansion of the main wound for manual extraction of the cataract. Having a wound posterior to the corneal plane would allow faster wound healing, eliminate induced astigmatism, and give a better visual outcome. There were many cases during my residency and, later, resident cases that I staffed, in which we were thankful to have spent the time creating that tunnel.
Later in my career, as phaco machines and handpieces continued to become more efficient, even the dense lenses were able to be emulsified; however, the next day, the corneal endothelium showed the stress it bore, with corneal edema that was slow to resolve over the next few weeks.
Instrument Removes Challenges
It wasn’t until I first used the miLoop to divide the nucleus of a dense cataract that I felt that I could truly approach these mature lenses in the same way I do moderate cataracts—the same preparation, same anesthesia, and same time for visual recovery. I still remember the last scleral tunnel I created in one of my private cases for a dense cataract. The tunnel was something I so rarely performed that it took me just as long to make the main wound as it did to perform the entire cataract extraction!
My confidence with mature lenses has grown since becoming proficient with the miLoop and they have become more routine. The miLoop allows me to approach even a dense white cataract with intracameral anesthesia and a clear corneal incision but, as with any instrument, it’s important to realize its limitations.
COVID-19 and Back to Basics
Since the COVID-19 pandemic quarantine in New York, I have noticed many more mature cataracts, which is a phenomenon that requires further study and reporting. In the meantime, it has offered me an opportunity to perform many more of these surgeries with great success. My first case after the quarantine was a young woman whose cataract matured quickly. The capsule was adherent to the cortex, and I ended up using retinal microscissors to complete the capsulorhexis. For this case, I used my traditional divide and conquer technique, keeping in mind that the anterior capsulorhexis may not be as strong as one created by a continuous tear and thus is not ideal for the miLoop.
The More You Do, The better You Become
I have now tried many different approaches to dense cataracts, and no two cases are the same. No one technique or instrument works for every mature lens—each requires its own modifications. This proficiency and understanding develop over time. The more cases you do, the better you will perform, and the more consistent your results will be.