
This article describes three technologies that allow different types of noninvasive correction, including cylindrical and spherocylindrical, after cataract surgery.
REFRACTIVE INDEX SHAPING
How it works. Perfect Lens developed refractive index shaping (RIS) technology for the noninvasive adjustment of IOL power with a femtosecond laser. The laser uses green light and operates at a much lower energy level than is required for ablation or cutting. The technology may be used with acrylic IOLs already on the market.1,2
The application of laser energy induces a chemical reaction in a specific area within the substance of the IOL, simultaneously causing an increase in hydrophilicity and a decrease in the refractive index. At the molecular level, hydrolysis occurs when the IOL polymeric material is exposed to laser energy and hydrophilic functional groups form. Because the lens is in an aqueous medium, hydrogen bonds form between water molecules and the new functional groups while the integrity of the polymeric material is preserved. A phase-wrapping algorithm is used to shape an ultrathin, high-diopter lens layer within the existing IOL. Several other corrections may be made later using areas above or below the initial area.
Laser treatment is fast, and the patient requires only topical anesthesia. The IOL changes do not occur immediately, because they require complete hydration of the treated area within the lens. This is reached at different time points, depending on the base material of the IOL. Astigmatic treatment may be performed after the IOL has stabilized inside the capsular bag. Even high levels of customized toricity can be achieved (Figure 1).
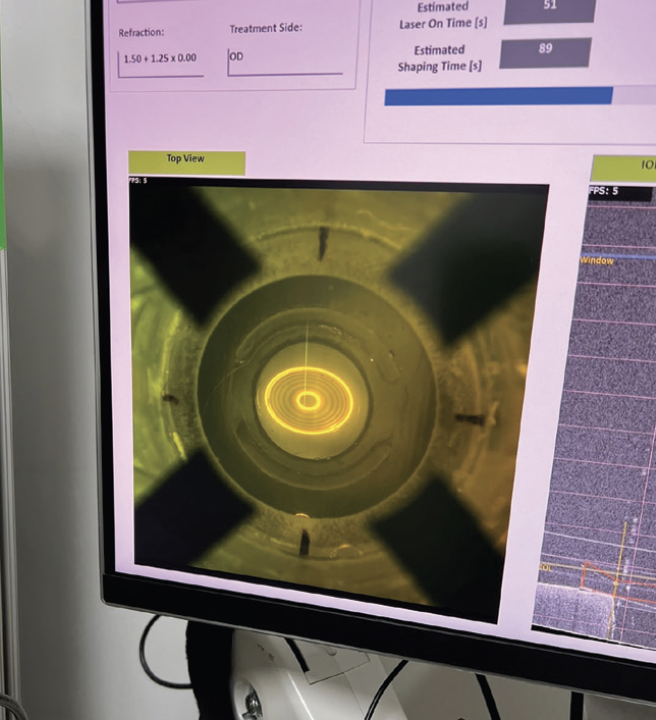
Figure 1. A spherocylindrical pattern is applied with RIS to a one-piece hydrophobic acrylic lens in vitro.
Courtesy of Ruth Sahler, Perfect Lens
Research results. My colleagues and I evaluated the IOL power, modulation transfer function, light transmission, and light scattering of a blue light–filtering IOL before and after power adjustment with a femtosecond laser.3 Ten one-piece yellow hydrophobic acrylic IOLs were used in the study. All measurements were performed on hydrated IOLs. The lenses were also evaluated with light microscopy before and after laser adjustment. A mean power change of -2.037 D was associated with a modulation transfer function change of -0.064 and a light transmittance change of -1.4%. Back light scattering increased within the IOL optic in the zone corresponding to the laser treatment at levels that were not expected to be clinically significant. Treated areas within the optic could be well appreciated with light microscopy without damage to the IOLs.
We also evaluated the uveal and capsular biocompatibility of IOL power adjustment with a femtosecond laser in vivo in a rabbit model.4 Six rabbits underwent phacoemulsification and bilateral implantation of a commercially available hydrophobic acrylic IOL. The postoperative power adjustment was performed 2 weeks after IOL implantation in one eye of each rabbit. The animals were observed clinically for 2 weeks and then euthanized. Slit-lamp examinations performed after laser treatment found that small gas bubbles formed behind the lenses and disappeared within a few hours. No postoperative inflammation or toxicity was observed in the laser-treated eyes, and postoperative outcomes and histopathologic examination results were similar to those in untreated eyes. The power measurements showed that the changes in power obtained were consistent and within ±0.10 D of the target. Similar findings were observed in a long-term rabbit study (follow-up period of 6 months after laser treatment; unpublished data).
The first human clinical trial of the RIS technology started in Panama but was interrupted by the COVID-19 pandemic. It resumed in 2022. All patients enrolled to date received monofocal one-piece hydrophobic acrylic lenses (Tecnis Monofocal 1-Piece, Johnson & Johnson Vision). Postoperative laser treatments, usually lasting approximately 89 seconds, were performed for spherical, cylindrical, and spherocylindrical corrections. No postoperative drops were administered after laser treatment, only artificial tears.
Data are available for seven patients treated in 2022 and show that the desired refraction change was close to the actual refractive change obtained when no movement was observed during treatment. Changes to the flexible silicone interface were made to constrain involuntary eye movement during RIS. There was no loss of contrast sensitivity or change in color perception. A patient questionnaire on visual disturbances found no complaints of halos or glare after laser treatment (unpublished data).
Additional application. RIS technology could be used in IOL manufacturing. Full customization of monofocal IOLs with RIS could be performed at the manufacturing facility, and the lenses could be shipped before surgery. Potential advantages include a low cost for premium optical features, fewer safety limitations (because the lens would not be inside the eye at the time of laser treatment), and lower inventory costs. Manufacturers, moreover, would use their own proprietary materials without having to alter their manufacturing processes for different types of lenses.
LASER-INDUCED REFRACTIVE INDEX CHANGE
How it works. Clerio Vision developed laser-induced refractive index change (LIRIC) technology. Treatment with a 405-nm wavelength femtosecond laser system induces refractive index changes. In principle, LIRIC and RIS are similar.5-8 To date, LIRIC technology has been tested on the cornea, contact lenses, and IOLs. Most studies have focused on its use in the cornea. After cataract surgery, LIRIC treatment can inscribe a variety of optical patterns within the cornea or the IOL to correct residual refractive errors, including astigmatism.
The laser operates at a pulse energy that is 100 to 1,000 times lower than the flap-cutting regimen currently used in femtosecond LASIK. No tissue is ablated during LIRIC. The refractive index is changed through modification of the mixture of collagen and water within the treated micrometer-sized region of the corneal stroma. Specifically, the application of laser energy modifies the collagen matrix so that the collagen fibrils are more densely packed.
Avoiding tissue ablation obviates concern about corneal ectasia. LIRIC preserves the original corneal curvature and avoids epithelial remodeling and the subsequent regression seen in the early days of laser refractive surgery. The low pulse energies, moreover, have been shown histologically to preserve the stromal nerves, which could reduce the incidence of postoperative dry eye. All of this provides the potential to repeat treatment on the same eye without significantly changing the cornea.
The corneal procedure takes just under 90 seconds. The goal is to reduce treatment time to less than 20 seconds.
Research results. In vivo animal experiments found that the effect of LIRIC treatment persisted in the cornea for up to 2 years. No detrimental effect on corneal endothelial cells was observed.7,8
In the first clinical study (n = 27 patients) conducted to establish safety outcomes,5 no eyes exhibited inflammation or a wound-healing response. All corneas were clear after treatment. There were no signs of haze, scarring, endothelial damage, or opacity. A flat-applanation patient interface similar to flat applanation for cutting a LASIK flap was used. Patients recovered in approximately 24 hours and did not need routine topical antibiotics or steroids for the first 5 to 7 days following treatment.
LIRIC contact lens research is currently focused on presbyopia correction (eg, embedding diffractive bifocal or trifocal patterns in the contact lens; Figure 2) and myopia control. The use of other wavelengths, such as 520 nm (green) and 1,035 nm (near infrared), is also being explored.

Figure 2. A diffractive multifocal pattern is written inside a contact lens with LIRIC.
Courtesy of Len Zheleznyak, Clerio Vision
LIGHT ADJUSTABLE LENS
How it works. The Light Adjustable Lens (LAL; RxSight) is a three-piece silicone lens with modified-C PMMA haptics, a 6-mm optic, and an overall length of 13 mm. The medical-grade UV light–filtering silicone optic contains a light-activated photoinitiator and mobile silicone macromers. The application of UV light (365 nm) causes these macromers to polymerize in the treated area. Subsequent diffusion of unpolymerized macromers to the treated region changes the LAL’s shape and thus its power (Figure 3).9 Light treatment can adjust the LAL’s spherical power from -2.00 to +2.00 D and its cylindrical power from -0.50 to -2.00 D in 0.25 D increments. Once the desired refractive outcome has been reached, it is locked in with a final light treatment.

Figure 3. The mechanism of power change with the LAL.
Courtesy of Roy Freeman, RxSight
The first light treatment is typically performed 2 to 3 weeks after LAL implantation. Three to five total light treatments, each lasting approximately 90 seconds and separated by approximately 3 days, are usually required. The patient wears proprietary UV-protective glasses until the lock-in treatment is performed. The latest-generation LAL features an ActivShield UV-protective layer in the anterior part of the lens that helps protect against unwanted UV exposure. This may eliminate the possibility of uncontrolled UV polymerization due to patient noncompliance with instructions to wear UV protection, but clinical studies are required.
Clinical experience. According to RxSight, more than 700 surgeons implant the LAL regularly. For a firsthand account of one surgeon’s clinical experience, see Benefits of a Light Adjustable Lens for Astigmatic Eyes.
CONCLUSION
The FDA approved the LAL in 2017, but RIS and LIRIC technologies are currently under investigation. It remains to be seen if and how the application of these technologies changes astigmatism management in the future.
1. Bille JF, Engelhardt J, Volpp HR, et al. Chemical basis for alteration of an intraocular lens using a femtosecond laser. Biomed Opt Express. 2017;8(3):1390-1404.
2. Sahler R, Bille JF, Enright S, Chhoeung S, Chan K. Creation of a refractive lens within an existing intraocular lens using a femtosecond laser. J Cataract Refract Surg. 2016;42(8):1207-1215.
3. Nguyen J, Werner L, Ludlow J, et al. Intraocular lens power adjustment by a femtosecond laser: in vitro evaluation of power change, modulation transfer function, light transmission, and light scattering in a blue light-filtering lens. J Cataract Refract Surg. 2018;44(2):226-230.
4. Werner L, Ludlow J, Nguyen J, et al. Biocompatibility of intraocular lens power adjustment using a femtosecond laser in a rabbit model. J Cataract Refract Surg. 2017;43(8):1100-1106.
5. Leonard C. LIRIC: A Novel LVC Treatment. Review of Ophthalmology. February 10, 2022. Accessed May 30, 2023. https://www.reviewofophthalmology.com/article/liric-a-novel-lvc-treatment
6. Savage DE, Brooks DR, DeMagistris M, et al. First demonstration of ocular refractive change using blue-IRIS in live cats. Invest Ophthalmol Vis Sci. 2014;55(7):4603-4612.
7. Wozniak KT, Butler SC, He X, Ellis JD, Knox WH, Huxlin KR. Temporal evolution of the biological response to laser-induced refractive index change (LIRIC) in rabbit corneas. Exp Eye Res. 2021;207:108579.
8. Zheleznyak L, Butler SC, Cox IG, et al. First-in-human laser-induced refractive index change (LIRIC) treatment of the cornea. Invest Ophthalmol Vis Sci. 2019;60:9:5079-5079.
9. Ford J, Werner L, Mamalis N. Adjustable intraocular lens power technology. J Cataract Refract Surg. 2014;40(7):1205-1223.
Benefits of a Light Adjustable Lens for Astigmatic Eyes
By J. David Stephens, MD

The importance of astigmatism correction at the time of cataract surgery increases as patient demand for excellent postoperative vision grows. Predicting the magnitude and axis of astigmatism, however, is limited by the variable accuracy of posterior corneal measurements and, more importantly, postoperative healing.
Traditionally, residual astigmatism has been managed with spectacles, limbal relaxing incisions, corneal refractive surgery, and IOL exchange. Most of these approaches entail additional surgical risk, and all of them require time and may leave the patient and physician frustrated. An advantage of a Light Adjustable Lens (LAL; RxSight) is it allows astigmatism to be corrected based on the patient’s postoperative manifest refraction instead of preoperative calculations. The foldable three-piece LAL is composed of photosensitive silicone, and multiple refractive adjustments with UV light may be performed before the final power is locked in.
MY APPROACH TO ASTIGMATISM CORRECTION
Primary candidates. I favor the LAL in situations where the accuracy of the IOL calculation is questionable (eg, patients with a history of refractive surgery) and where the goal is sharp visual acuity with minimal risk of postoperative glare and halos. Approximately 2.00 D of sphere or cylinder can be corrected per light treatment. I find that the maximum amount of total astigmatism correction with an LAL is about 2.50 to 3.00 D.
In a review of the first 54 eyes at my practice to receive an LAL with ActivShield technology, the magnitude of astigmatism on manifest refraction decreased from 0.79 ±0.70 D before the first light treatment of the LAL to 0.13 ±0.29 D at the final lock-in, usually 6 weeks after surgery (Figure 1).1 Of the 54 eyes, 26 (48%) had previously undergone corneal refractive surgery, and 79% achieved a result within ±0.25 D of the spherical refractive target.

Figure 1. The mean absolute decrease of the magnitude of astigmatism from before the first light treatment of the LAL to the final lock-in.
High regular astigmatism. I find the LAL to be the most accurate option for eyes with low to moderate regular astigmatism. I favor toric IOLs for eyes with high regular astigmatism because the axis and magnitude tend to be easier to predict.
Irregular astigmatism. The LAL may be a good option in this situation, particularly if the patient is unable to tolerate a rigid gas permeable contact lens to attain optimal vision. The implantation of an LAL could potentially diminish their postoperative astigmatism to a degree sufficient to optimize vision without requiring a contact lens.
CASE EXAMPLE: KERATOCONUS
A 67-year-old man with long-standing keratoconus presented for a cataract surgery evaluation. The patient had attempted to wear rigid gas permeable and scleral contact lenses in the past but could not tolerate either owing to difficulty with the lenses’ daily removal. He was therefore motivated to reduce his manifest refraction as much as possible.
The patient’s glasses prescription had not changed significantly during the past several years. Topography showed inferior steepening consistent with keratoconus in each eye (Figure 2). Measurements with the IOLMaster (Carl Zeiss Meditec) showed 3.60 D of astigmatism OD and 7.50 D OS (Figure 3).

Figure 2. Topography showing inferior steepening consistent with keratoconus in the right (A) and left (B) eyes.
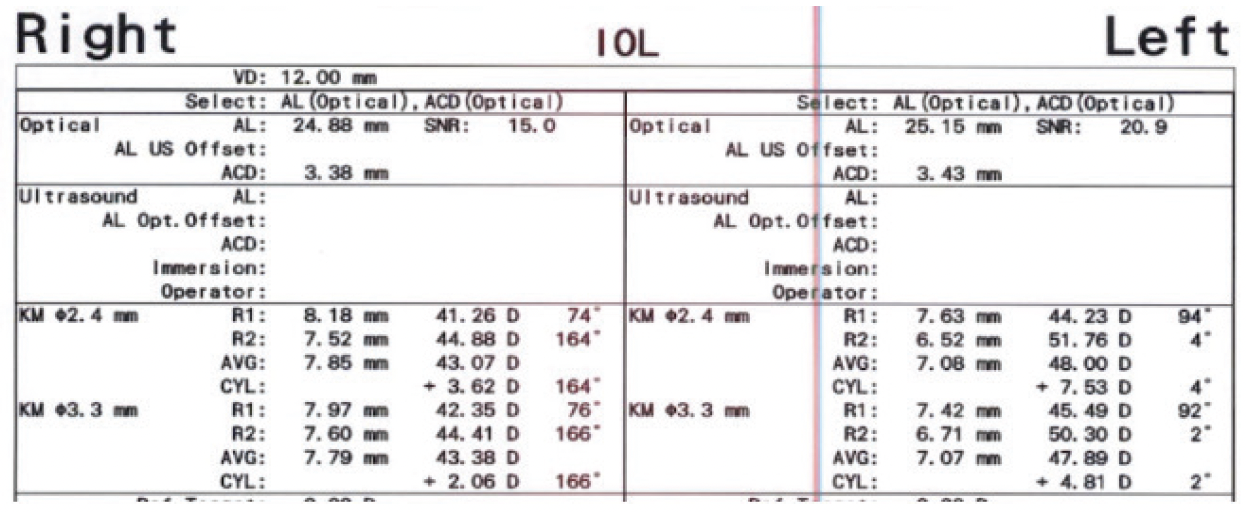
Figure 3. Measurements with the IOLMaster showed 3.60 D of astigmatism OD and 7.50 D OS.
IOL calculations were performed with the Kane KCN formula, and a plano result was targeted in both eyes. An LAL was implanted bilaterally.
At the time of the first light adjustment, the patient’s BCVA was 20/25-2 OD with a manifest refraction of +2.25 -2.75 x 75º. His UCVA was 20/300 OS, and his BCVA was 20/30 OS with a manifest refraction of +4.00 -2.75 x 65º. After the third and final light adjustment of the LAL in each eye, his BCVA was 20/25-2 OD with a refraction of +0.50 -1.00 x 100º. His UCVA was 20/80 OS, and his BCVA was 20/30 OS with a refraction of +1.75 -2.25 x 100º. Interestingly, the magnitude of astigmatism in the left eye was significantly lower than preoperative biometry had suggested, which underscores the unpredictable impact of the posterior cornea in eyes with ectasia or an irregular cornea.
The patient was able to drive without spectacle correction. He wore prescription readers for near tasks.
1. Stephens JD. Paper presented at: The Caribbean Eye Meeting; February 4–7, 2022; Rio Grande, Puerto Rico.




