The prevalence of chickenpox, which is caused by varicella-zoster virus (VZV), varies globally. The United States began vaccinating children against VZV in 1995. Most people in the country who are 40 years of age or older have had chickenpox. Australia and Canada, which also implemented VZV immunization, have rates similar to the rate in the United States, but the prevalence is higher in the United Kingdom and France, for example, where the vaccine has not been widely recommended.
At a Glance
- Approximately 1 million people per year develop herpes zoster (commonly referred to as shingles) in the United States alone. Between 10% and 20% of these individuals develop herpes zoster ophthalmicus (HZO).
- HZO can cause severe, long-lasting eye disease and vision loss, chronic pain, and in rare cases, stroke.
- The Zoster Eye Disease Study is evaluating whether 1 year of suppressive therapy with a low dose of valacyclovir can delay or reduce complications in patients with HZO. Enrollment ended on December 31, 2022. Results are expected in 2024.
As the human body ages, the immune system weakens, and latent VZV may be reactivated and cause herpes zoster, better known to the public as shingles. Approximately 1 million people per year develop herpes zoster in the United States, and 10% to 20% of them develop herpes zoster ophthalmicus (HZO). The incidence of HZO increases with age and has been reported to vary across racial groups.1 The condition can cause severe, long-lasting eye disease and vision loss, chronic pain, and, in rare cases, stroke (Figure). In a retrospective cohort study of nearly 900 individuals with acute HZO conducted at a single center in New Zealand, approximately one in 10 developed moderate or severe vision loss, primarily from corneal scarring.2 Older age, immunosuppression, and uveitis were associated with severe, permanent vision loss.
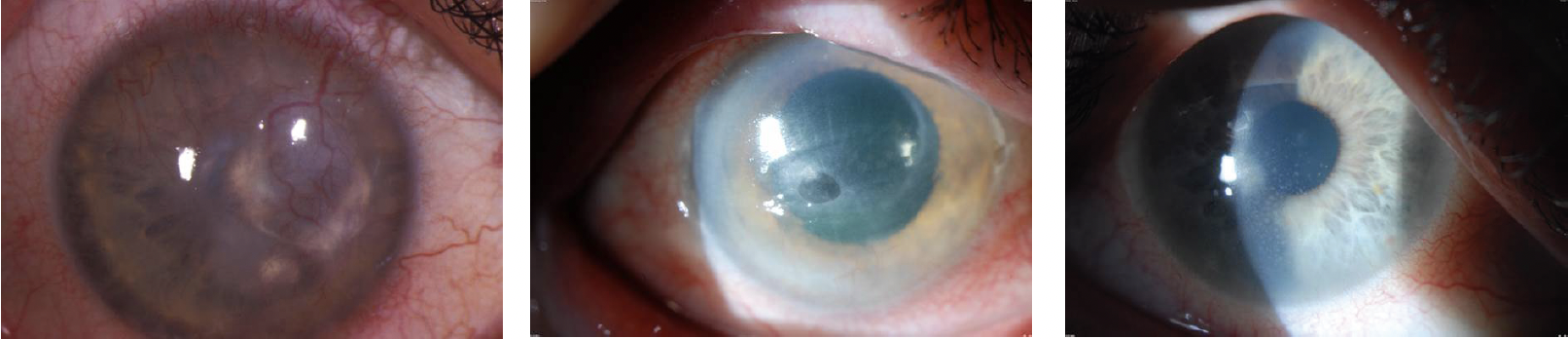
Figure. Herpes keratitis.
Courtesy of Christopher J. Rapuano, MD
“Loss of vision does not have to be severe to be very limiting,” Elisabeth J. Cohen, MD, told BMC, parent company of CRST and other vision publications including Glaucoma Today, Retina Today, MillennialEYE, and Modern Optometry. Dr. Cohen is the vice chair for academic affairs for the department of ophthalmology at NYU Langone Health in New York City. She developed HZO in 2008 and, after 1 year, experienced a permanent reduction in visual acuity to 20/40. “That loss of vision reduced my depth perception to the point where I had to give up doing corneal surgery and taking optimal care of cornea patients in the office, which involves good depth perception.”
HZO causes pain in most patients. “Typically, people have terrible pain for 4 to 6 weeks,” Dr. Cohen said. “It can be very disabling and very hard to treat.” She added that 20% to 25% of patients experience postherpetic neuralgia, which is defined as pain lasting longer than 3 months after disease onset. People are more likely to develop this chronic pain syndrome if they are 60 years of age or older at the time of HZO onset. “The pain is not just in the eye,” Dr. Cohen clarified. “It’s in the distribution of the [trigeminal] nerve.” The pain can be so difficult for individuals to endure, she said, that it may cause suicidal thoughts or actions.
The challenges of HZO and Dr. Cohen’s personal experience with the condition are the genesis of the Zoster Eye Disease Study (ZEDS). She is the study chair and a principal investigator for ZEDS, which is evaluating whether 1 year of suppressive therapy with a low dose of valacyclovir can delay recurrences or prevent chronic disease and/or lessen chronic pain in individuals with HZO. She received the Cornea Society’s prestigious Castroviejo Medal in 2015 for her work to prevent HZO and efforts to conduct this clinical trial.
IMPETUS FOR THE STUDY
As noted earlier, active VZV infection causes HZO. Suppressive antiviral therapy has proven effective for the treatment of eye disease caused by another form of herpes—herpes simplex virus (HSV). Specifically, the Herpetic Eye Disease Study (HEDS) Acyclovir Prevention Trial (APT) found that 1 year of treatment with twice-daily oral acyclovir 400 mg reduced the rate of recurrent ocular HSV disease by 45%. Treatment was most beneficial for patients who had a history of HSV stromal keratitis.3
“When I was experiencing HZO, no one thought about treating me with a prolonged course of a low-dose antiviral drug,” Dr. Cohen recalled. “After vision loss knocked me out of my normal profession, I said, ‘Why don’t we try treating this just like we do HSV?’” She got to work on her first grant proposal.
ZEDS DESIGN AND METHODOLOGY
ZEDS is modeled after HEDS. Funded by a grant from the National Eye Institute, ZEDS is a multicenter, double-masked, placebo-controlled, randomized clinical trial. It is enrolling immunocompetent individuals who are 18 years of age or older, were diagnosed with HZO with a typical rash, and have experienced an episode of active epithelial or stromal keratitis and/or iritis within 1 year of enrollment. Participants are randomly assigned (1:1 ratio) to receive either 1,000 mg oral valacyclovir or placebo daily for 1 year. The study protocol, however, allows participants who experience new or worsening disease to receive open-label antiviral treatment.
The primary outcome measure is the time to first occurrence of new or worsening dendriform epithelial keratitis, stromal keratitis with or without ulceration, endothelial keratitis, or iritis due to HZO during the 12 months of treatment. Participants are observed for 6 months after cessation of the study medication to determine if there is a persistent treatment benefit. Results will be compared between groups randomly assigned in a double-masked fashion to receive either valacyclovir or placebo. The incidence, severity, and duration of postherpetic neuralgia will also be evaluated and compared between groups.4
WHAT TO EXPECT FROM ZEDS
Enrollment. Approximately 60 clinical centers in the United States, Canada, and New Zealand are participating in the trial, and more than 500 individuals had been enrolled at press time. Enrollment ended on December 31, 2022. According to Dr. Cohen, results will be available in 2024 (see For More Information).
“We don’t know if the treatment works,” Dr. Cohen remarked. “This study gives us a unique opportunity to see if prolonged, low-dose antiviral treatment reduces recurrent and/or chronic eye disease as well as the chronic pain syndrome.”
Results. Dr. Cohen anticipates that the first report on ZEDS findings will be released within 6 months of when study treatment ends because data on participants are being completed on a rolling basis.
FUTURE DIRECTIONS
Broader applications. If ZEDS demonstrates that antiviral treatment can reduce the incidence, severity, and duration of postherpetic neuralgia, therapy may be generalizable to individuals who have herpes zoster in other locations to reduce postherpetic neuralgia, Dr. Cohen said.
A persistent benefit. The Centers for Disease Control and Prevention (CDC) recommends administering a high dose of an oral antiviral for 7 to 10 days to treat acute zoster. What is currently unknown is whether suppressive treatment for 1 year would improve outcomes, Dr. Cohen noted. In a study of herpetic eye disease, the recurrence rate of ocular HSV disease after antiviral treatment ceased was the same as before it was initiated.2 A persistent posttreatment benefit was not observed, she said. In contrast, Dr. Cohen expressed cautious optimism that therapy may have a persistent benefit for HZO because of differences between it and ocular HSV.
“HSV goes on and off over many, many years, [whereas herpes] zoster tends to have a rough downhill course initially and then peters out over years,” she commented. Patients with herpes zoster may require long-term topical steroid therapy, but attacks or flares decrease.
Vaccination. The CDC recommends that all adults 50 years of age and older and people 19 years of age and older who are or will become immunocompromised receive the recombinant zoster vaccine, adjuvanted (Shingrix; GSK). Should individuals with HZO receive Shingrix as well? Approximately 6% of people with herpes zoster experience a second episode.5 The CDC recommends immunization after the episode of herpes zoster has resolved but does not specify when. Health Canada recommends waiting 12 months after an episode before vaccination. Dr. Cohen and her colleagues used to recommend vaccination 1 to 3 years after an episode, but she currently favors 12 months.
For More Information
Eye care professionals interested in more information about the Zoster Eye Disease Study are encouraged to contact the study’s coordinating center by calling (844) 698-9337 or sending an email to zeds.cramonitor@nyulangone.org. A list of active participating clinical centers in the United States, Canada, and New Zealand may be viewed by clicking here.
A concern about immunization, she stated, is that it could trigger ocular inflammation—something that was observed with an earlier vaccine for herpes zoster and was reported with the current vaccine.6,7 She says that ZEDS will collect data on participants who receive Shingrix and compare the rates of worsening eye disease by treatment group.
1. Borkar DS, Tham VM, Esterberg E, et al. Incidence of herpes zoster ophthalmicus: results from the Pacific Ocular Inflammation Study. Ophthalmology. 2013;120(3):451-456.
2. Niederer RL, Meyer JJ, Liu K, Danesh-Meyer HV. Herpes zoster ophthalmicus clinical presentation and risk factors for loss of vision. Am J Ophthalmol. 2021;226:83-89.
3. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med. 1998;339(5):300-306.
4. Cohen EJ, Hochman JS, Troxel AB, Colby KA, Jeng BH; ZEDS Trial Research Group. Zoster Eye Disease Study: rationale and design. Cornea. 2022;41(5):562-571.
5. Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86(2):88-93.
6. Lehmann A, Matoba A. Reactivation of herpes zoster stromal keratitis after HZ/su adjuvanted herpes zoster subunit vaccine. Ophthalmology. 2018;125(11):1682.
7. Jabbour S, Shekhawat NS, Chen A, Woreta FA. Presumed herpes zoster ophthalmicus reactivation following recombinant zoster vaccination. Cornea. 2021;40(2):248-250.




