CASE PRESENTATION
A 73-year-old White woman presents for a cataract surgery evaluation. The patient’s history is significant for bilateral radial keratotomy (RK). Her BCVA is +1.00 +1.50 x 165º = 20/80 OD and +3.00 +2.75 x 165º = 20/50 OS.
A clinical examination finds eight RK incisions, a 2+ nuclear sclerotic cataract, and 1+ cortical changes in the right eye and 12 RK incisions, a 2+ to 3+ nuclear sclerotic cataract, and 2+ to 3+ cortical changes in the left eye (Figure 1). There is a lamellar macular hole in the right eye. Macular OCT imaging of the left eye is normal. Figure 2 shows preoperative imaging of the left eye with the Pentacam (Oculus Optikgeräte).
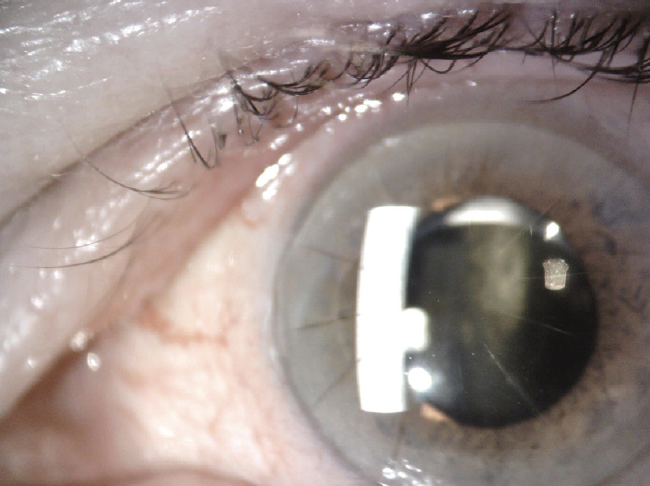
Figure 1. Cataract and RK incisions in the left eye.
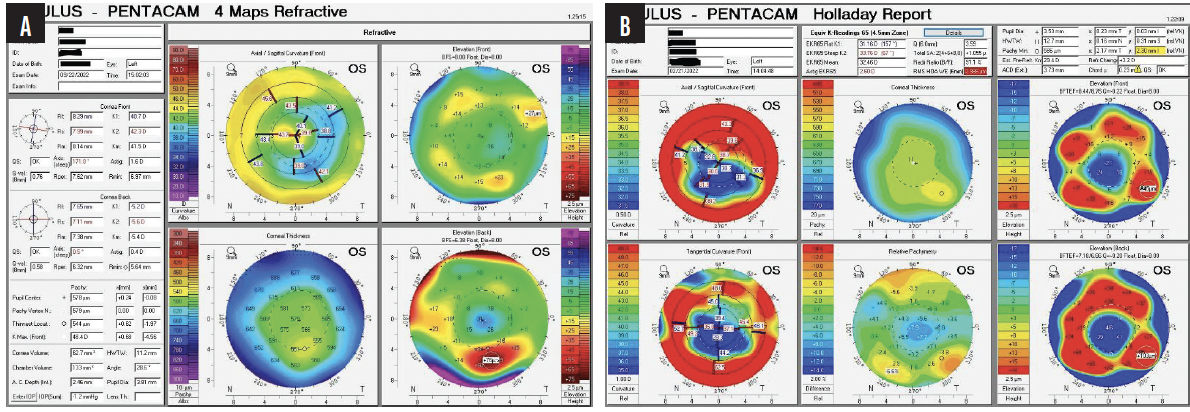
Figure 2. Preoperative tomography (A) and Holladay report (B) for the left eye.
The patient wishes to improve the vision in her left eye with cataract surgery before undergoing retinal surgery on her right eye. She desires spectacle independence at distance (Figure 3).

Figure 3. Preoperative biometry measurements for the left eye.
Which IOL would you choose for the left eye? If you would consider a toric model, at what axis would you place the IOL? Would you obtain further testing? Would you perform cataract surgery on the right eye before or after the retinal procedure? How would you counsel the patient?
— Case prepared by Audrey R. Talley Rostov, MD

H. BURKHARD DICK, MD, PHD, FEBOS-CR
In the coming years, cataract surgeons are likely to see a growing number of patients like this one—individuals who underwent refractive surgery in its early days. The current patient’s priority is cataract surgery on her left eye. She is aware that the visual prognosis for her right eye is poor due to the macular hole. The macula of the left eye appears to be healthy, but the cornea has 12 incisions and significant irregularity, with differences in the center of up to 4.00 D. Imaging with the Pentacam shows 1.60 D of astigmatism that is almost completely within the photopic pupillary opening plus two hot spots, the smaller of which is located toward the temporal side and the larger of which is more nasal. Based on the topographic findings, I am disinclined to offer a toric IOL; the astigmatism does not extend beyond the pupil, the meridians are not orthogonal to each other, and the entire line-of-sight complex seems to be slightly dislocated. Attention must be paid to chord mu—0.23 on the Holladay Report—which indicates that the patient is not looking through the geometric center of the cornea.
With 12 RK incisions, the chance of ripping open the cornea intraoperatively cannot be disregarded. This would complicate surgery and require the placement of sutures, which could induce astigmatism.
The formerly highly myopic patient is primarily interested in good distance visual acuity, so a multifocal or extended depth of focus IOL is not indicated. Instead, I would recommend an IC-8 Apthera (Bausch + Lomb). The pinhole effect can correct up to 1.50 D of astigmatism. The lens should provide the patient with stable distance visual acuity irrespective of any residual astigmatism.
Angles alpha and kappa would be measured. The IOL calculation would not use information from the IOLMaster 500 (Carl Zeiss Meditec) because the device extrapolates corneal radii and anterior chamber depth based on image analysis. The eye would be measured instead with the IOLMaster 700, which comes with swept-source OCT combined with Pentacam HD–based ray tracing using the physiologic pupil size as a setting. Otherwise, ray tracing is misleading because it measures the entire corneal area. When choosing the IOL power, I would target mild myopia to shift the defocus curve slightly toward myopia. This should enhance the patient’s intermediate and near vision without compromising her distance vision.
If necessary, cataract and retinal surgery would be performed in one session to minimize the patient’s anxiety. The combined approach can be easier to undertake in Europe than some other countries because many European cataract surgeons also perform retinal surgery.
It is imperative to underpromise and overdeliver in the current situation.

DAVID R. HARDTEN, MD, FACS
The case presents two major issues.
No. 1: Concurrent retinal pathology and cataract. In situations where the retina surgeon believes that retinal surgery may not be required (ie, for a nonprogressive pathology such as an epiretinal membrane), I typically address the cataract first. Aggressive therapy with a steroid and NSAID is administered before and after surgery. Thereafter, if the patient’s visual acuity is inadequate, then the retinal pathology is addressed with a second procedure.
Not all lamellar macular holes require surgery. If, however, the view through the cataract is adequate and the retinal pathology is the major contributor to the patient’s poor vision, then I would address the retinal pathology first.
No. 2: Severe irregular corneal astigmatism. The amount of irregular astigmatism dictates my choice of IOL. Patients’ level of satisfaction with their vision before a cataract developed guides expectations. If they had good UCVA and best spectacle-corrected visual acuity, it is often possible for them to achieve spectacle independence after cataract and IOL surgery.
The current patient has significant irregular astigmatism. I doubt that she had good vision without a contact lens in the past. I would therefore counsel her that her postoperative vision will be sharpest with a scleral contact lens. Toricity in the IOL makes fitting a scleral lens more difficult because the intraocular astigmatism is no longer neutralized by the contact lens. If the patient’s best spectacle-corrected visual acuity was good before the cataract developed, then I would recommend a Light Adjustable Lens (LAL; RxSight) for the left eye. Light adjustments of the IOL would be delayed for 2 to 3 months until the corneal curvature stabilizes because it takes longer for edema-related fluctuation to return to baseline in patients who have a history of RK. The RK incisions are tightly spaced and near the limbus, so a scleral incision would be used for cataract surgery.

SHERI ROWEN, MD, FACS
Patients with a history of RK have been knocking on the doors of our practices for years. Optimizing the refractive results of cataract surgery in this population is challenging because it is impossible to obtain accurate readings of the cornea, especially if the patient’s vision fluctuates throughout the day. The complexity of the current case is compounded by a macular hole in one eye.
Many years ago, I began implanting a Crystalens AO or Trulign Toric IOL (both from Bausch + Lomb) or a plano aspheric monofocal IOL (enVista IOL model MX60E or toric model MX60T; Bausch + Lomb) in post-RK eyes to avoid adding aberrations to the optical system. One problem is that these lenses can address only approximately 2.00 D of astigmatism. Many of the corneas, however, are highly irregular. I recently operated on one that had more than 6.00 D of induced astigmatism caused by an astigmatic keratotomy that crossed the RK incisions. The contralateral eye had 4.00 D of cylinder. Both eyes had more than 20 RK incisions. The patient now has functional spectacle-free driving vision (20/25 in the better-seeing eye) and is exceedingly happy with the LAL.
My current lens of choice for these patients is an LAL, which I would implant bilaterally in this case with scleral incisions. The Barrett True-K formula would be used for the IOL calculation to determine the closest approximation of spherical power. Light adjustments would be performed after the cornea demonstrates stability (at least 6–8 weeks postoperatively). An advantage of the LAL is that it will provide a clear view for the retina surgeon when they operate on the lamellar macular hole. If silicone oil is likely to be required, however, an LAL could be an issue.
The LAL is basically a monofocal IOL, but many of my patients achieve an extended range of vision with the postoperative light adjustments. My RK patients have been highly satisfied with their results thus far. I have been able to treat up to 4.00 D of astigmatism with correct placement of the axis according to the patient’s actual refraction. I plan to use the IC-8 Apthera to address irregular corneal astigmatism as well when the lens becomes more widely available in the United States, where I practice.

WHAT I DID: AUDREY R. TALLEY ROSTOV, MD
The patient and I had a long discussion about IOL choices, including waiting for the Apthera, which is not yet widely available in the United States, where I practice. I also consulted with the retina surgeon in my practice. A decision was made to proceed with cataract surgery on the right eye before the retinal procedure to maximize the patient’s visual acuity and improve the view for the retina surgeon if macular hole repair is subsequently performed.
Biaxial laser cataract surgery using scleral incisions was performed. I find the option of creating a customized capsulotomy that has alignment nubs or tabs with the Lensar Laser System (Lensar) facilitates excellent alignment of a toric IOL. It is important to bear in mind that discontinuities in the capsulotomy may occur where an RK incision is located and to remove the tissue carefully with microforceps so that the alignment tabs are preserved.
A dispersive OVD was injected to maintain the anterior chamber during the procedure. An enVista toric IOL (model MX60T200) was implanted and aligned at 165º. The refractive target was -1.00 D. I find the IOL’s aberration neutral platform to be advantageous in eyes with irregular corneas, eyes with a high angle alpha or kappa, and situations in which the risk of IOL decentration is increased.
Postoperatively, the patient’s BCVA was 20/40+2 with a manifest refraction of -1.25 + 0.50 x 170º OD. She will receive an Apthera IOL in her left eye.




