CASE PRESENTATION
A 49-year-old man presented with blurry vision and difficulty reading. The patient had undergone myopic LASIK surgery 23 year ago. On examination, his UCVA was 20/30 OD and 20/70 OS, and his uncorrected near visual acuity (UNVA) was 20/80 OU. Autorefraction was near emmetropia in each eye (Figure 1). Imaging with the Pentacam (Oculus Optikgeräte) showed an inferiorly decentered LASIK ablation in the left eye (Figure 2).
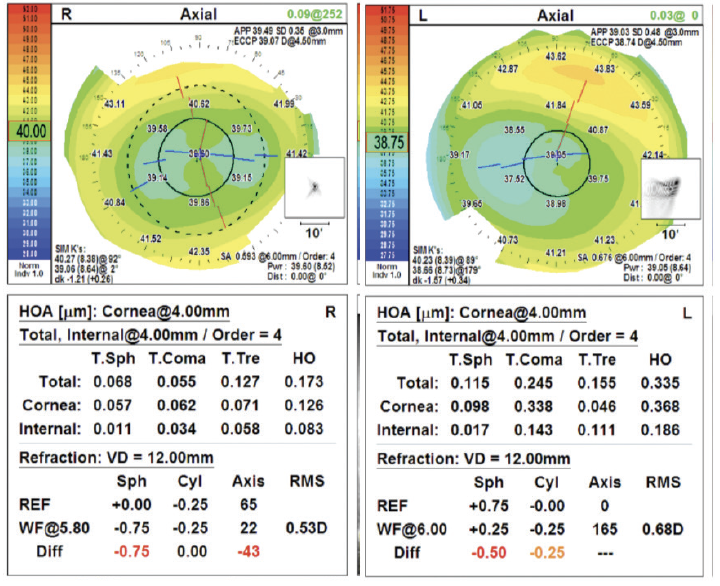
Figure 1. Autorefraction of both eyes with the OPD III (Marco Ophthalmics).
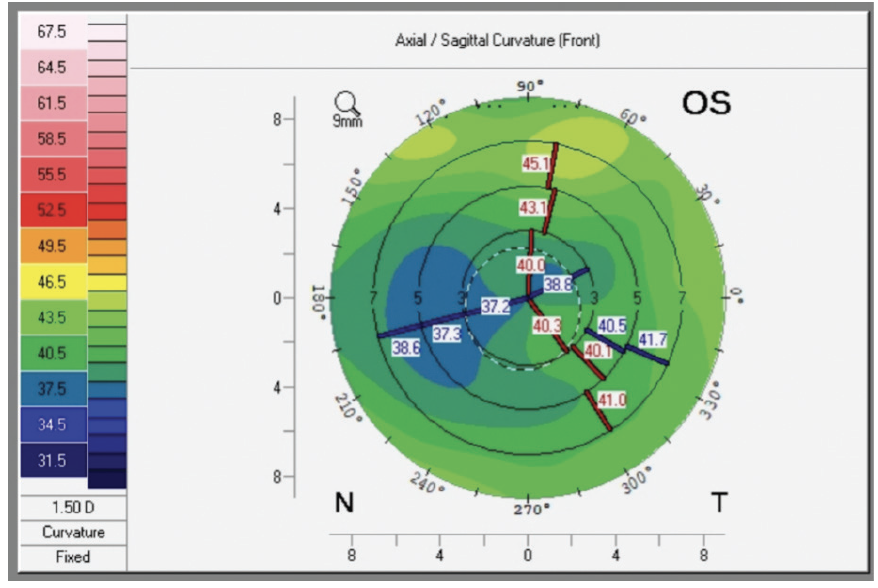
Figure 2. Imaging of the left eye with the Pentacam shows an inferiorly decentered LASIK ablation.
Upon further examination with slit-lamp biomicroscopy, the crystalline lenses were determined to be at dysfunctional lens syndrome stage 2. An overrefraction with a rigid gas permeable (RGP) contact lens was considered but had not been performed. The patient was informed that the prior refractive surgery could limit his visual potential. Given the topographic findings, a blended vision strategy with the Light Adjustable Lens (LAL; RxSight) was recommended. He decided to proceed, and surgery was performed without complications.
One month postoperatively, his uncorrected distance visual acuity is 20/63 OD and 20/126 OS. His corrected distance visual acuity is 20/25 with a manifest refraction of +1.00 -0.75 x 060º OD and 20/40 with a manifest refraction of +1.00 -2.50 x 058º OS. His UNVA is 20/32 OD and 20/63 OS.
The patient is satisfied with his potential distance vision but unhappy with his potential near targets given a corrected distance visual acuity of only 20/40 OS. No treatments of the LAL with the Light Delivery Device (RxSight) have been performed yet. How would you proceed?
—Case prepared by Jonathan D. Shader, DO, and Luke Rebenitsch, MD

VANCE THOMPSON, MD, FACS
We do not have the patient’s preoperative manifest refraction or BCVA, so my response involves some assumptions. Because the ablation in the left eye was decentered, an overrefraction with an RGP contact lens would be performed to help determine how much of the blur is corneal and how much is lenticular. With an otherwise normal examination, if the manifest refraction improves from say 20/150 or 20/100 to 20/30 with the overrefraction, then most of the blur is corneal. In this scenario, I would recommend the implantation of an IC-8 Apthera (Bausch + Lomb) in the left eye, which should correct corneal and lenticular blur. The small-aperture optics should improve the patient’s distance and near vision.
I do all I can preoperatively to treat corneal blur whenever possible and allow 3 to 6 months of healing, remodeling, and stabilization to occur before I perform cataract surgery. The LAL is an excellent option for eyes with a history of corneal refractive surgery. As with corneal refractive surgery, however, lens-based enhancements require an accurate manifest refraction. It cannot be assumed that the manifest refraction is accurate when the patient’s BCVA is reduced to the degree it is in this case.
He has already undergone cataract surgery. An overrefraction with an RGP contact lens should be performed, and amblyopia must be ruled out. I would also review the patient’s history. If he does not have amblyopia and experiences no improvement with an overrefraction, then it must be determined whether he has been diligent about wearing UV-protective goggles outside. If not, irregular polymerization of the LAL is possible, especially if it is an early model without ActivShield Technology (RxSight's UV protector).
If the patient’s vision improves significantly with the overrefraction, topography-guided LASIK would be performed off label to treat the coma only. Again, the patient’s manifest refraction may not be accurate because of his reduced BCVA, so I would not attempt to treat lower-order aberrations. Reliable and repeatable corneal aberration measurements, however, would help me feel comfortable about treating higher-order aberrations (HOAs). The intervention could optimize the optical zone if his vision is crisp with an RGP contact lens overrefraction.
Once refractive stability is achieved and the manifest refraction is accurate, light adjustments to the LAL would be performed. To my knowledge, a 193-nm excimer laser does not interact with an unlocked LAL with ActivShield Technology, but I would double-check with RxSight.

O. BENNETT WALTON IV, MD, MBA
When the shape of the cornea is atypical after refractive surgery, I want to know if the patient’s quality of vision was good in each eye immediately postoperatively. In other words, I am interested in whether and to what extent shape has affected quality of vision. If the current patient was happy with the quality of vision in his left eye, the next step is to achieve the optimal trade-off with a mini-monovision strategy when performing light treatments of the LAL. I find that the lock-in process helps determine a patient’s subjective sweet spot. If the patient was never happy with his left eye’s quality of vision, we would discuss the use of RGP contact lenses, likely a scleral or hybrid design. The tear-lens effect can compensate better for a decentered ablation than any spectacle or IOL adjustment can. I tell individuals with irregular corneas that their vision will be better with an RGP contact lens than with a non–contact lens method.
Once patients have a sense of their postoperative vision, then we can choose to treat only the sphere and plan for either a contact lens or spherocylindrical correction in the LAL. I have the advantage of working with optometrists who are highly skilled with scleral and hybrid contact lenses.
Here, no refractive target for the near (left) eye has achieved adequate UNVA. One strategy is for the patient to wear a contact lens on his left eye with a near target. Another option is to perform topography-guided LASIK or PRK, which in the past would have required the LAL to be locked in before refractive surgery. If the goal is to normalize the cornea before locking in the LAL, then I would have a discussion with engineering colleagues to ensure that excimer laser energy will be blocked by the LAL’s ActivShield Technology. A third option is to exchange the IOL in the left eye for an IC-8 Apthera, which would likely lessen the optical impact of the HOAs and elongate the focal range.
I would discuss the options with the patient and assess his comfort level with various levels of invasiveness.


WHAT WE DID: JONATHAN D. SHADER, DO, AND LUKE REBENITSCH, MD
After counseling, the patient was interested in pursuing any option that could deliver his best potential vision, even if a prolonged surgical course was required. Given the decentered ablation (Figure 2) and amount of associated HOAs, we recommended the off-label use of topography-guided PRK with the WaveLight EX500 excimer laser (Alcon) and explained that it would involve a 3-month healing period. We also emphasized that it would be important for the patient to wear sunglasses whenever exposed to UV light, regardless of the LAL’s ActivShield Technology. He chose to proceed with PRK on his left eye before undergoing light adjustment treatment of the LAL with the Light Delivery Device and light adjustment treatment of the LAL in his right eye before PRK to optimize his distance vision (plano target) during his postoperative recovery.
Refractive stability was achieved in the left eye around 3 months after PRK. The subsequent light adjustment treatments to the LAL proved challenging. The targeted refraction was 1.50 D. Three light treatments were required to achieve a final refraction of -1.75 D (Figure 3).
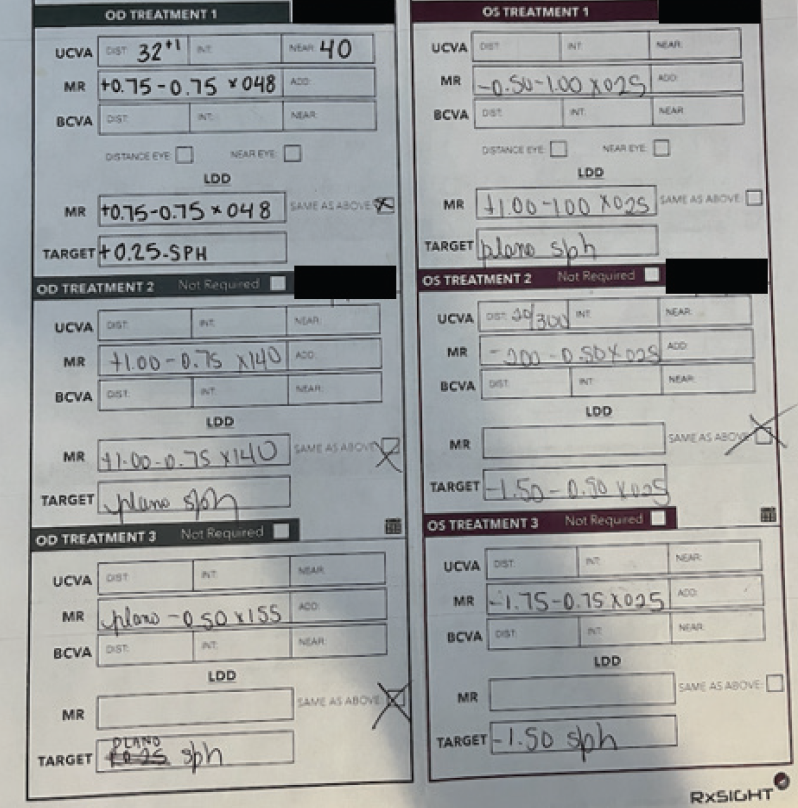
Figure 3. LAL treatments.
The patient’s final refraction was plano OD and -1.75 D OS. His binocular uncorrected distance visual acuity and UNVA were 20/20 OU. He was happy with his result.
In hindsight, we would perform an RGP contact lens overrefraction to help determine the contribution of corneal HOAs to the patient’s vision loss. Based on the information, we would also pursue topography-guided PRK before implanting an LAL. We have since used this strategy in similar situations with success.




