Dry eye disease (DED) affects an estimated 36 million people in the United States.1 About 18 million people are diagnosed with DED,1 and of those, 1.1 million are treated with prescription medication.2 DED is shown to negatively impact many aspects of life including mood, leisure activities, and work, in addition to causing ocular pain and discomfort.3 Cynthia Matossian, MD, FACS, and Jai G. Parekh, MD, MBA, FAAO, hosted a webinar to discuss the underlying mechanisms and diagnosis of the disease.
The Tear Film and Ocular Surface Homeostasis
The ocular tear film consists of an aqueous-mucin layer, which contains fluid and soluble factors produced by the lacrimal glands and mucin secreted by the goblet cells, that is covered by the lipid layer.4 These layers contain a combination of nutrients needed to maintain a healthy ocular surface.4 Ocular surface homeostasis depends on a balance of tear production and evaporation, and when this is disrupted, DED is usually present.5
Disrupted ocular surface homeostasis occurs when tear evaporation exceeds tear generation, whether that is due to insufficient tear production or faster than normal tear evaporation.5,6 Evaporative tear loss often overlaps with aqueous deficiency and is the underlying etiology in nearly 90% of DED cases (Figure 1).7 Excessive tear evaporation can be caused by one or more contributing factors, such as lipid deficiency, lifestyle, and aqueous and mucin deficiency,5,8 which lead to surface desiccation, tear film dysregulation/instability, tissue damage, and inflammation (Figure 2).9 Desiccation stress causes corneal damage including barrier disruption, loss of tight junctions, and epithelial apoptosis.10 Squamous metaplasia, loss of goblet cells, and CD4 T cell infiltration may also occur.11
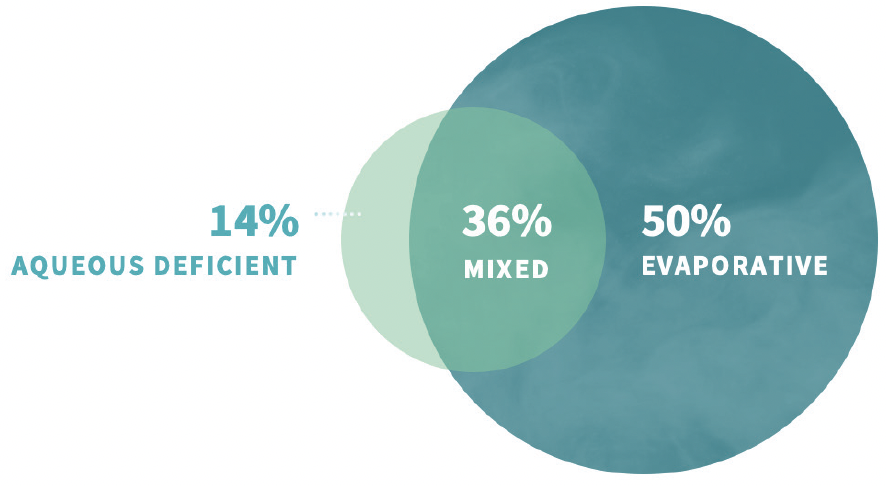
Figure 1. Dry eye disease etiology.5
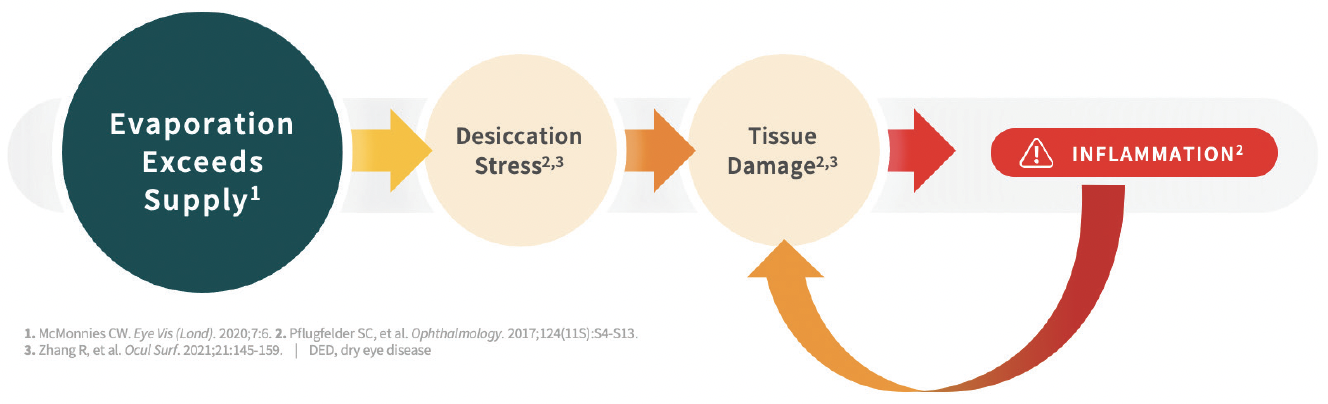
Figure 2. Excessive tear evaporation leads to inflammation, damage, and disease progression.
Meibomian gland dysfunction is a principal contributor to evaporative DED.5 If the meibum secreted is reduced or altered, tears lose their aqueous-protecting lipid layer, leading to more rapid tear film disruption and evaporation.12
Lifestyle factors exacerbate the evaporative state.8 “Most of us do not blink enough. We do not sleep enough, we have high stress levels, and we look at our phones all the time, which affects our blink dynamic,” said Dr. Parekh. Full blinking is required to distribute tears from the lacrimal glands and lipids from the meibomian glands over the ocular surface.13 Screen time can cause our blink rate to drop from 22 blinks per minute to seven blinks per minute.14 “It is rare for us to have pristine, healthy tear film because the dry eye state can be triggered by environmental factors, seasonal changes, or a bad day,” said Dr. Parekh.
“Dry eye is more than just a nuisance. It can cause a ripple effect impacting quality of life, work, and mood.3 In the past, we did not have adequate ways to diagnose or treat it. Now we do,” said Dr. Matossian.
Diagnosing DED
DED is common in candidates for ocular surgery, and the relationship between signs and symptoms of DED is often discordant.15 This makes diagnosis challenging. One large study found that characteristic symptoms of DED were not reported in nearly 60% of patients with other objective evidence of DED.16 However, another large cohort study showed that patients with concomitant factors such as chronic pain, atopic disease, and depression tended to report more severe DED symptoms than would be suggested by clinical signs. Particularly, patients with lower self-perceived overall health report more severe symptoms versus signs. “Patients are notoriously inconsistent with reporting symptoms of DED, both understating and overstating severity. Many patients with DED have not been treated or diagnosed properly. There is a huge chasm between those categories. Our job as experts is to mitigate and reduce that, to really focus on the patient journey. This could have a considerable impact on candidates for cataract or refractive surgery,” said Dr. Parekh.
Despite the discordant reports of symptoms, Drs. Matossian and Parekh encourage a simple and systematic process for diagnosing DED. “Take the time to look for DED. Do not skip over it and go right to lens opacity level. We have objective tests like tear osmolarity, MMP-9 testing, and meibography. You do not need to have all three. Start with one and get comfortable with it, and add other tests to your toolbox later. I also stain every cataract consult to look for DED. I show them the staining results and let them know that they have a curable cataract, but that DED is lifelong and requires treatment. Having objective numbers can help in counseling patients who doubted that they had DED. Later, use these numbers to show them treatment is working,” said Dr. Matossian.
“Excessive evaporation is the main consequence of meibomian gland dysfunction. Without intervention, the cycle of tear evaporation leading to hyperosmolarity then ocular surface damage and inflammation is never ending.17 Look at the meibomian glands. Hold the lid down, lift the lid up, and push on it. You can do this with a cotton tip applicator or your finger. Check to see if the orifices are open, the quality of the meibum, and whether the meibum is coming out. You do not need any fancy equipment,” said Dr. Matossian.
“For a more objective approach to diagnosis, meibomian gland imaging is a helpful diagnostic tool that we can use to determine a meiboscore (Figure 3). Graded from zero to three, the meiboscore number increases with telltale signs of gland loss, alterations, and changes to architecture. These are powerful images that show us the pathology. We can use these to help patients understand the level of their disease,” said Dr. Parekh.
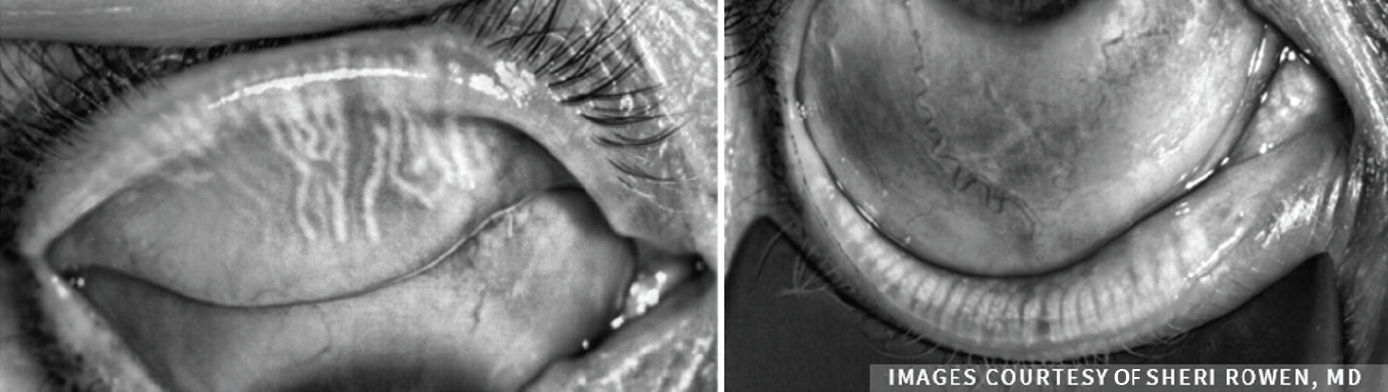
Figure 3. Assessing meibomian gland morphology.
Treating DED
DED management must meet individual needs and address root causes to the degree possible. Some existing DED treatments have been associated with high rates of dissatisfaction and discontinuation due to factors such as burning and stinging associated with initial use; long-term use of preservatives; and lag time among patients between initiation of treatment and symptomatic relief.18 However, they do work in many patients and are the best option until there is an FDA-approved medication that targets the root cause of evaporative DED. “Use both objective and subjective tools to diagnose, and use the treatments we have to help those patients,” said Dr. Matossian.
1. 2020 Dry Eye Products Market Report: A global Analysis for 2019 to 2025. Market Scope. https://www.market-scope.com/pages/reports/250/2020-ophthalmic-landscape-report-global-analysis-for-2019-to-2025-april-2021#reports.
2. IQVIA data.
3. Dana R, Meunier J, Markowitz JT, Joseph C, Siffel C. Patient-reported burden of dry eye disease in the United States: results of an online cross-sectional survey. Am J Opthalmol. 2020 Aug;216:7-17.
4. Pflugfelder SC, Stern ME. Biological functions of tear film. Exp Eye Res. 2020;197:108115.
5. McMonnies CW. Aqueous deficiency is a contributor to evaporation-related dry eye disease. Eye and Vis (Lond). 2020;7:6. doi: 10.1186/s40662-019-0172-z.
6. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438-510.
7. Lemp M, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-8. doi: 10.1097/ICO.0b013e318225415a.
8. Wolffsohn JS, Wang MTM, Vidal-Rohr M, et al. Demographic and lifestyle risk factors of dry eye disease subtypes: A cross-sectional study. Ocul Surf. 2021;21:58-63. doi: 10.1016/j.jtos.2021.05.001.
9. Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: What we know and future directions for research. Ophthalmology. 2017;124(11S):S4-S13. doi: 10.1016/j.ophtha.2017.07.010.
10. Perez VL, Stern ME, Pflugfelder SC. Inflammatory basis for dry eye disease flares. Exp Eye Res. 2020;201:108294. doi: 10.1016/j.exer.2020.108294.
11. Zhang R, Pandzic E, Park M, Wakefield D, Di Girolamo N. Inducing dry eye disease using a custom engineered desiccation system: Impact on the ocular surface including keratin-14-positive limbal epithelial stem cells. Ocul Surf. 2021;21:145-159. doi: 10.1016/j.jtos.2021.04.006.
12. Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: The role of gland dysfunction in dry eye disease. Ophthalmology. 2017 Nov;124(11S):S20-S26. doi: 10.1016/j.ophtha.2017.05.031.
13. Al-Mohtaseb Z, Schachter S, Lee BS, Garlich J, Trattler W. The relationship between dry eye disease and digital screen use. Clin Ophthalmol. 2021;15:3811-3820. doi: 10.2147/OPTH.S321591.
14. Tsubota K, Nakamori K. Dry eyes and video display terminals. N Engl J Med. 1993;328(8):584. doi: 10.1056/NEJM199302253280817.
15. Trattler WB, Majmudar PA, Donnenfeld ED, McDonald MB, Stonecipher KG, Goldberg DF. The prospective health assessment of cataract patients’ ocular surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423-1430. doi.org/10.2147/OPTH.S120159.
16. Vehof J, Smitt-Kamminga NS, Nibourg SA, Hammond CJ. Predictors of discordance between symptoms and signs in dry eye disease. Ophthalmology. 2017;124:280-286. doi: 10.1016/j.ophtha.2016.11.008.
17. Baudouin C, Messmer EM, Aragona P, et al. Revisiting the vicious cycle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100:300–306. doi:10.1136/bjophthalmol-2015-307415.
18. White DE, Zhao Y, Ogundele A, et al. Real-world treatment patterns of cyclosporine ophthalmic emulsion and lifitegrast ophthalmic solution among patients with dry eye. Clin Ophthalmol. 2019;13:2285-2292. doi: 10.2147/OPTH.S226168.
©2023 Bausch & Lomb Incorporated or its affiliates. UBL.0056.USA.22





