The gram-positive anaerobe Clostridium botulinum produces the neurotoxic protein botulinum toxin, which, in 2019, commanded a $5 billion market. Botulism outbreaks during the Napoleonic Wars shined a spotlight on this extremely lethal toxin in the 1800s, but it was not identified and extracted in a laboratory until the 1920s, when the growth of another billion-dollar industry—the canned food industry—made botulism a costly public health hazard. Researchers developed a significant understanding of its mechanism of action over the next few decades, and ophthalmologists undertook the first experiments on its clinical use in the 1960s. FDA approval of the use of botulinum toxin for strabismus and blepharospasm followed in 1983. Over the next few years, as people noticed the welcome side effects of botulinum toxin in patients treated for blepharospasm, the off-label cosmetic use of this toxin grew in popularity. In 2002, the FDA approved Botox (onabotulinumtoxinA, Allergan) for cosmetic use in the management of glabellar furrows.1
This article can serve as a starting point for ophthalmologists who are interested in growing their practice by offering aesthetic services.
An Introduction to Toxins
Any practitioner with a thorough knowledge of the periocular musculature’s functional anatomy will be able to deliver excellent and consistent outcomes to patients regardless of the specific botulinum toxin preparation used, as long as he or she is knowledgeable about and experienced with the behavior of the chosen preparation. Patients, on the other hand, will often demonstrate a strong preference for one preparation over another.
Mechanism of action. Botulinum toxin acts on the presynaptic terminal of the neuromuscular junction by reversibly inhibiting the release of acetylcholine, which results in temporary muscle weakening or paralysis.
Side effects. In the hands of experienced practitioners, all currently available formulations of botulinum toxin share a nearly identical and small list of side effects, mainly consisting of injection discomfort, temporary erythema, occasional bruising at the injection site, and short-term postinjection headache. If the toxin’s tissue diffusion is excessive, however, the potential side effects affect the muscles and are longer lasting. The most common of these is eyelid ptosis (and various other versions of facial asymmetry), which can last for several weeks depending on the amount of overtreatment and can be extremely disconcerting to the patient.
Although severe hypersensitivity or botulism is extremely rare, a careful history, including previous allergies, should be taken for all patients considering toxin injection. All potential side effects should be discussed with patients before the procedure.
Contraindications. The list of contraindications for botulinum toxin is of critical importance and must be studied carefully by anyone interested in offering toxin injections in the practice. Pregnant and breastfeeding women should never be treated with botulinum toxins.
Dosing. Botulinum toxin dosing for rhytid management slightly varies depending on the patient’s age, sex, muscle mass, and toxin tolerance. Manufacturer recommendations do not necessarily reflect these individualities. As with all clinical procedures, physicians are likely to develop individual treatment strategies as they gain experience.
Uses
Although initially considered to be a predictable cosmetic treatment only for the upper face, botulinum toxin treatment has recently gained popularity and achieved excellent results in the lower face.
Most oculoplastic surgeons practicing cosmetic medicine in Europe seem to focus on the treatment of the upper face.
Carefully placed intra- or perimuscular injections (Figure) weaken the effect of periocular muscles, diminishing glabellar lines (procerus and corrugator muscles), forehead rhytids (frontalis muscle), crow’s feet (lateral orbicularis oculi muscle), or the so-called bunny lines on the dorsolateral nose (nasalis). In the upper face, botulinum toxin is also used as a perioperative adjunct, as well as for chemical brow elevation and widening of the eyes.
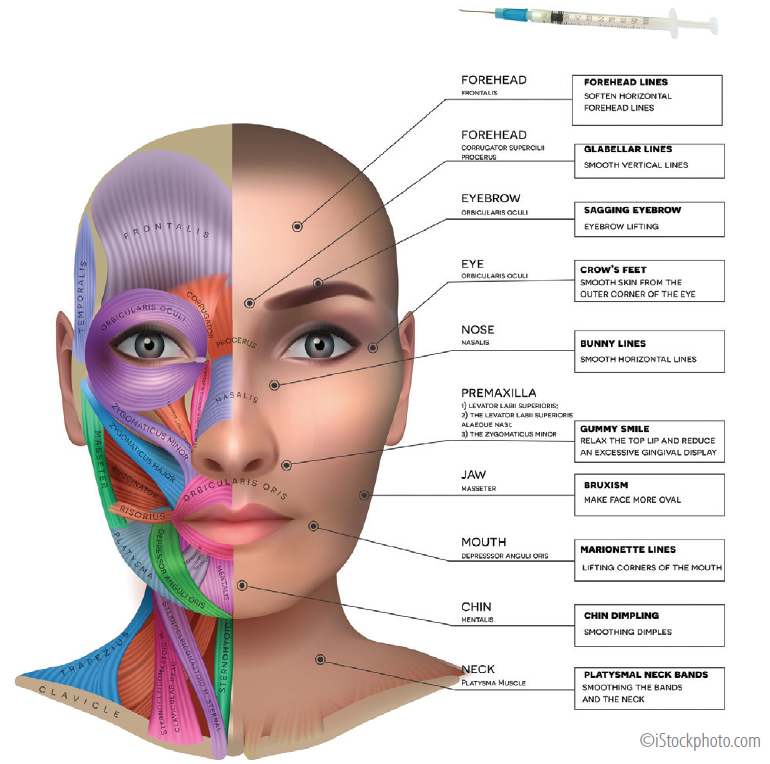
Figure. Facial muscle anatomy and neurotoxin injection sites, target muscles, and the effects produced.
Botulinum Toxin Preparations
Currently, seven structurally similar serotypes of botulinum toxin (types A–F) are known. Only some, however, have any effect on humans. Of those that do, only types A and B are approved for use by the FDA. All cosmetically used botulinum toxins are type A.
Any ophthalmologist interested in cosmetic medicine should be aware of five botulinum toxin preparations, which are addressed here in order of their emergence on the market.
Botox
Botox is the original and most widely used and investigated botulinum toxin formulation available, representing approximately 80% of the global toxin market. It is indicated and licensed for—among many other noncosmetic indications—the treatment of glabellar lines and crows feet.
Most studies have shown that the onset of the desired effect occurs approximately 5 days after injection. Early effects can be seen sooner, at around 2 or 3 days after injection, and continued improvement occurs for up to 2 weeks with a median overall duration of effect of approximately 3.9 months.
In practice, these times can vary due to both the treatment’s success and the patients’ perception.
Dysport
Dysport (apobotulinumtoxinA, Galderma) is approved for treating glabellar lines and crow’s feet and uses completely different unit with the Botox:Dysport unit ratio quoted as 1:2.5. Although some trial results suggest that this product has a quicker onset of action and a shorter duration of effect than Botox, in the absence of exhaustive head-to-head studies, Dysport and Botox should be thought to have comparable times to effect and durations of effect.
Studies in patients with cervical dystonia have indicated that Dysport may diffuse more to surrounding tissues than Botox, potentially leading to unwanted side effects.2,3 For this reason, Dysport is sometimes not selected for periocular cosmetic cases. The product is unsuitable for patients with milk allergies or lactose intolerance because it contains lactose and protein from cow’s milk.
Xeomin
Xeomin (incobotulinumtoxinA, Merz Aesthetics) was developed in Germany. Again, this botulinum toxin preparation behaves similarly to Botox and has a nearly 1:1 dose ratio.4,5 Unlike other commercially available preparations, Xeomin does not contain complexing proteins,6 which may reduce the risk of allergic reactions and natural resistance. Xeomin is often recommended for individuals demonstrating treatment tolerance and whose treatment with other agents is therefore losing effect.
Jeuveau
Jeuveau (prabotulinumtoxinA, Evolus) is the most recent addition to the family of available botulinum toxins. It was approved for use in the United States in 2019 and is indicated for temporary improvement in the appearance of moderate to severe glabellar lines in adults. Because Jeuveau has the same particle size as Botox, the two preparations should share similar efficacy and safety profiles, but supporting clinical data are limited.
DaxibotulinumtoxinA
DaxibotulinumtoxinA (RT002, Revance) is a potential game-changer. The agent is currently awaiting review for FDA approval. Three phase 3 trials have demonstrated a median duration of response of 24 weeks, which is significantly longer than that of its competitor neurotoxins. In terms of safety and tolerability, no novel concerns have been raised with this preparation.7
If or when approved, daxibotulinumtoxinA will likely be a desirable option for patients seeking less frequent treatment visits.
Take-Home Message
Botulinum toxin injections can offer safe and extremely rewarding outcomes for patients. As with all cosmetic interventions, the most important step is to listen carefully to patients’ wishes and hold careful discussions about how they align with what the treatment can offer.
1. França K, Kumar A, Fioranelli M, Lotti T, Tirant M, Roccia MG. The history of botulinum toxin: from poison to beauty. Wien Med Wochenschr. 2017;167(suppl 1):46-48.
2. Ranoux D, Gury C, Fondarai J, Mas JL, Zuber M. Respective potencies of Botox and Dysport: a double blind, randomised, crossover study in cervical dystonia. J Neurol Neurosurg Psychiatry. 2002;72:459-462.
3. Brefel-Courbon C, Simonetta-Moreau M, More C, et al. A pharmacoeconomic evaluation of botulinum toxin in the treatment of spasmodic torticollis. Clin Neuropharmacol. 2000;23:203-207
4. Sattler G, Callander MJ, Grablowitz D, et al. Noninferiority of incobotulinumtoxina, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown lines. Dermatol Surg. 2010;36(suppl 4):2146-2154.
5. Prager W, Wissmüller E, Kollhorst B, Böer A, Zschocke I. Treatment of crow’s feet with two different botulinum toxin type A preparations in split-face technique. Hautarzt. 2011;62(5):375‐379.
6. Nigam PK, Nigam A. Botulinum toxin. Indian J Dermatol. 2010;55(1):8-14.
7. Carruthers J, Solish N, Humphrey S, et al. Injectable daxibotulinumtoxinA for the treatment of glabellar lines: a phase 2, randomized, dose-ranging, double-blind, multicenter comparison with Botox and placebo. Dermatol Surg. 2017;43:1321-1331.