NOEL ALPINS, AM, FRANZCO, FRCOPHTH, FACS
A concept in astigmatic treatment that many refractive surgeons find hard to digest is the nonzero target. When laser treatment is guided wholly by refractive (manifest refraction or wavefront refraction) or by corneal (topography-guided) parameters, surgeons believe they are targeting zero, but they are neglecting the other mode of treatment (corneal or refractive). It is almost as if a mirror were blocking their view of the effect of treatment on the other parameter of measurement.
The target-induced astigmatism vector is the link that connects the two treatment paradigms by considering and analyzing the astigmatic effect of both modes. This effect is rarely zero. Consider an example in which the astigmatic treatment was planned on the basis of the manifest refraction, and the refractive cylinder was +2.00 D x 20º (corneal plane). Corneal astigmatism, however, was 1.50 D @ 10º. This discrepancy (calculated vectorially to account for differences in magnitude and orientation) in preoperative corneal-refractive parameters is common, and it is known as ocular residual astigmatism (ORA).
Given this difference, it would not be possible to achieve zero astigmatism on the cornea because the planned treatment is +2.00 D x 20º (based on manifest refraction parameters). In theory, ablating +2.00 D x 20º onto a cornea with cylinder measured at 1.50 D @ 10º would leave 0.78 D x 40º ORA. This is what is termed the nonzero target. It would be only by chance—perhaps healing factors—that zero astigmatism would be achieved on the cornea in this case. The higher the nonzero amount (as quantified by the ORA), the worse the prospect of an outcome that will please the patient.
This unfortunate situation can be avoided in several ways. One of these is to identify the problem, if it exists, prior to performing surgery by quantifying the patient’s ORA at the time of counseling. It is a straightforward calculation with resources made available for free on websites such as www.assort.com.
Questions for the panel:
No. 1: Do you see a need to change the treatment plan for excimer laser surgery if preoperative differences exist between refractive cylinder and corneal astigmatism?
No. 2: Do you analyze refractive surgery astigmatism outcomes by corneal or refractive parameters, or do you consider both relevant?

PARAG A. MAJMUDAR, MD
Refractive astigmatism is the conglomeration of corneal astigmatism plus other, often described as lenticular, astigmatism. In many cases, the lenticular portion of astigmatism changes over time, often because of accommodation. For this reason, with the advent of topography-guided excimer laser ablation, there has been a trend toward focusing primarily on the corneal component of astigmatism when planning treatment. Topography-modified refraction (TMR) therefore often differs from clinical manifest refraction and involves using the corneal component of astigmatism to plan refractive surgery. Kanellopoulos has suggested that using the TMR may provide superior outcomes in lines of vision gained as well as total magnitude of residual astigmatism postoperatively.1
Using purely corneal astigmatic data may be problematic in that the global refractive outcome may be suboptimal if there is overcompensation for astigmatism, resulting in induced astigmatism or ORA. TMR often relies on an arbitrary compromise between the clinical refraction and topography-derived refraction, especially the astigmatic component.
Experience with topography-guided LASIK has shown that raised topographic features on the cornea have optical effects. In addition, Koch and colleagues have found that posterior corneal astigmatism also plays a role in the focusing of light.2 Behind the cornea, internal elements such as the lens can further change the path of light rays. It should not be surprising, therefore, that the magnitude and axis of anterior corneal astigmatism frequently differ from the magnitude and axis of manifest refractive astigmatism. In practice, the two differ more often than they agree. The manifest refractive astigmatism is the sum total of all of the refractive vectors that contribute to astigmatism as seen by the patient.
A mathematical approach is clearly required to determine what effect removing corneal irregularity in topography-guided ablation may have on overall refractive error. I have had the opportunity to work with the Phorcides Analytical Software designed by Mark Lobanoff, MD. It is designed to make these calculations more reproducible and less prone to subjective variation by analyzing the individual topographic elevation data and using vector analysis of the various sources of astigmatism to minimize ORA.
Phorcides was developed to perform the complex analysis of all sources of astigmatism within the eye. The program uses geographic imaging software to assess raised areas of corneal tissue, which create smaller slopes superimposed on the larger slope of the anterior corneal curvature. In geology, this formation is known as a talus, and this nomenclature has been adapted to corneal topography as well. Using lens theory and optical physics, the refractive cylinder contribution of the talus can be calculated.
The software program analyzes anterior and posterior curvature data from a Scheimpflug device, and it uses vector analysis to compare all the known vectors that contribute to refractive cylinder (corneal irregularity vector, anterior corneal astigmatism vector, posterior corneal astigmatism vector). The program compares this result to the manifest refraction of the patient, allowing calculation of any internal astigmatism vectors that reside between the posterior corneal curvature and the retina. The program assumes that topographic treatment will remove the corneal irregularity vectors. It then calculates how much anterior corneal astigmatism should be left after correction of the topography to counterbalance the posterior corneal and internal astigmatism vectors. Finally, Phorcides combines all the known and calculated vectors that contribute to astigmatism and recommends a treatment (Figure 1).
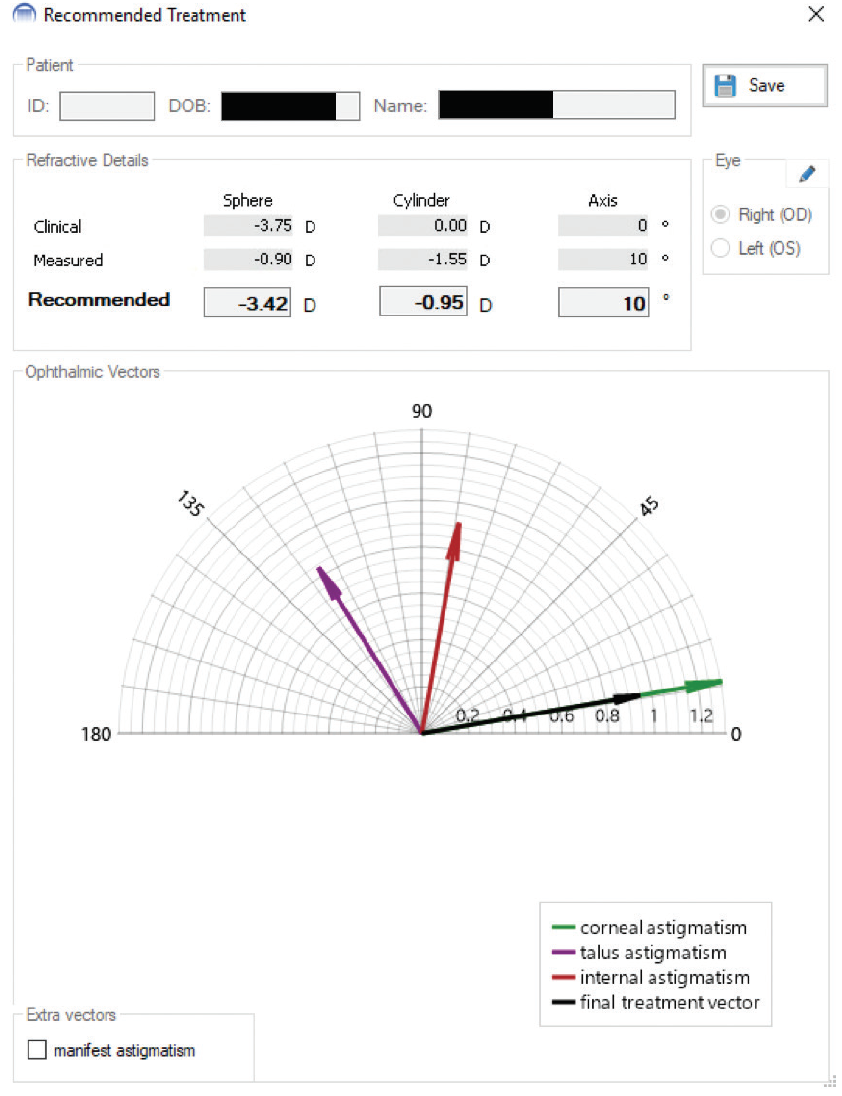
Figure 1. In this case, the manifest refraction (“Clinical”) shows no cylinder that is accepted by the patient at the phoropter, yet the corneal astigmatism (“Measured”) shows 1.55 D of cylinder. Looking at the vector diagram, the combined effects of the talus (corneal irregularity) and internal astigmatism vectors counterbalance the corneal astigmatism, which is why the patient chooses no astigmatic correction at the phoropter. The recommended treatment accounts for correction of residual astigmatism once the corneal irregularity is removed.
Courtesy of Parag A. Majmudar, MD
Early clinical results with Phorcides have been promising, exceeding those obtained when treating simply off the manifest refraction or the measured anterior astigmatism (TMR). More impressive, early Phorcides results are exceeding those found in the FDA study of Contoura Vision (Alcon). In the FDA study, patients were included only if their manifest and measured astigmatism were similar (within 10º or with magnitude differences < 0.75 D). In the current Phorcides studies, the results of which are expected to be published later this year, all eyes are included, even those with vast differences between manifest and measured astigmatism, according to Dr. Lobanoff.
Efforts of this sort will enable surgeons to predict the effect of topography-derived astigmatic ablation and, in turn, its effect on overall refractive condition. This may help to answer the question of what to do when surgeons face a patient with a discrepancy between manifest and corneal astigmatism in order to improve outcomes.
Regarding Dr. Alpins’ second question, the best measure of refractive surgery outcomes may be through analysis of refractive parameters as opposed to solely corneal parameters. It is well known that treatment of corneal astigmatism and especially higher-order aberrations affects lower-order aberrations such as sphere and cylinder. Although technology for assessing corneal aberrations is steadily improving, the most practical measure of success after refractive surgery will come from subjective manifest refraction, which will be an indicator of the global refraction, not just the corneal component.
KARL G. STONECIPHER, MD
For any refractive surgeon, astigmatism is a persistent challenge. For this article, I will limit my discussion to the treatment of regular astigmatism. If a patient’s corneal and refractive astigmatism match perfectly, the challenge is simple, but this is rarely the case with the patients I see daily. I look at the astigmatism of each patient and concentrate on refractive, anterior corneal, posterior corneal, and, of course, lenticular astigmatism.
My colleagues and I use several diagnostic devices in our clinic, including the OPD-Scan III (Nidek), Pentacam (Oculus Optikgeräte), WaveLight Topolyzer Vario Diagnostic Device (Alcon), and Advanced CustomVue (Johnson & Johnson Vision). Each of these technologies can either confirm or contradict the manifest and/or cycloplegic refraction obtained in the clinic.3-6 Now we can overanalyze the patient by reading too much into the diagnostics, especially when they conflict and do not make sense, and we have achieved excellent outcomes by treating the manifest refraction with wavefront-optimized protocols.7,8 If something does not make sense in terms of preoperative evaluation, we perform a wavefront-optimized treatment.
The goal, however, is to improve UCVA beyond what the patient saw with glasses or contact lenses. My colleagues and I are looking at ways to achieve outcomes better than 20/20 by using Phorcides software with the Vario topographer and the Pentacam tomographer in conjunction with the WaveLight excimer laser (Alcon). The software compares the manifest refraction, computed topography, and tomography using mathematics and vector analysis to calculate treatments (Figure 2). The Phorcides software is unique in that it finds the astigmatism vector created by topographic irregularities and compares it to both the cognitive refractive cylinder (CorT Total) found in the manifest and the actual measured corneal topographic astigmatism and posterior topographic astigmatism. Topographic irregularities can throw off the accuracy of Placido disc and Scheimpflug topographers based on their proximity to the corneal astigmatism. Greater precision and accounting for all sources of astigmatism, including that from topographic irregularities, leads to the most precise detection of ORA. The goal with the Phorcides software is to remove topographic irregularities while producing the perfect anterior corneal astigmatism to counterbalance ORA from posterior corneal and lenticular astigmatism that remains after LASIK. So far, we have been impressed with the outcomes.
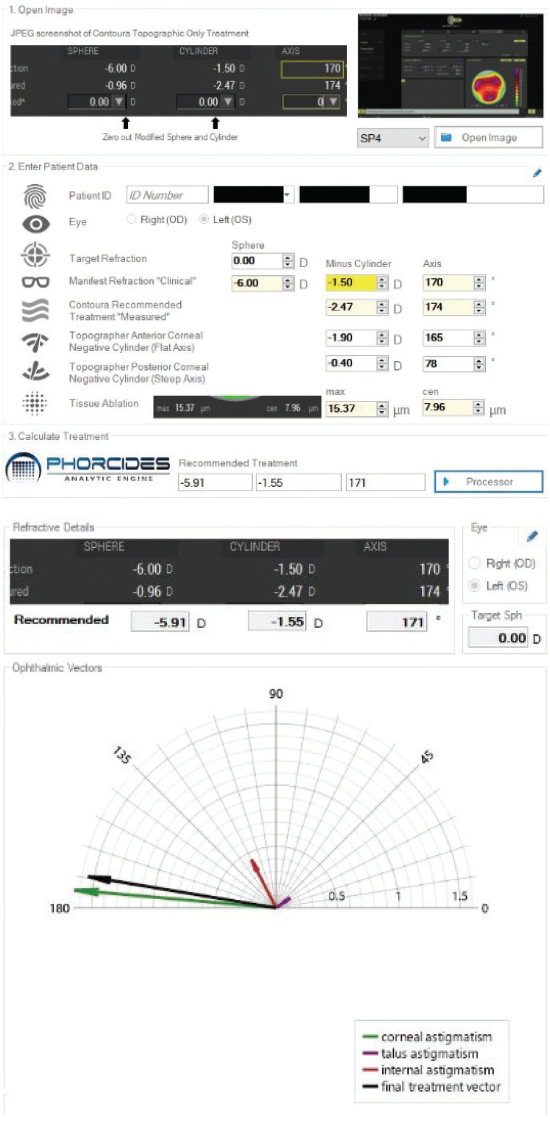
Figure 2. Phorcides software compares the manifest refraction, computed topography from the Vario, and tomography from the Pentacam to recommend a treatment (top). The underlying vector analysis of the corneal astigmatism, induced refractive change, internal astigmatism, and final treatment vector (bottom).
Courtesy of Karl G. Stonecipher, MD
We use a variety of parameters to measure our outcomes. Corneal and refractive outcomes are important, but, simply put, if the patient is happy and our enhancement rate is negligible, we do not complain too much. Currently, our enhancement rate with topography-guided treatments using the WaveLight excimer laser in a prospective trial is 0.2% (N = 1,712 eyes). That is for treatments of up to -9.00 D of myopia and up to 3.25 D of astigmatism by manifest refraction using wavefront-optimized software and topography-guided software. Outcomes using these treatment profiles are excellent.6 That said, analyzing corneal, refractive, and aberrometry outcomes is important. They all influence what the patient sees, which is the bottom line. Happy patient equals happy life.
A prospective contralateral eye study that we are conducting (N = 82 eyes of 42 patients) comparing results with the WaveLight Allegretto T-CAT treatment (Alcon) from the FDA study to those using Phorcides software are showing great outcomes as early as 1 day postoperatively (Figure 3). In this series, we have treated up to -7.63 D spherical equivalent with up to 3.25 D of manifest refractive cylinder. The average spherical equivalent was -3.72 ±1.58 D, and the average amount of astigmatism was 0.96 ±0.90 D. On postoperative day 1, UCVA was 1.42 ±0.28 (1.33 = 20/15) OU on average.
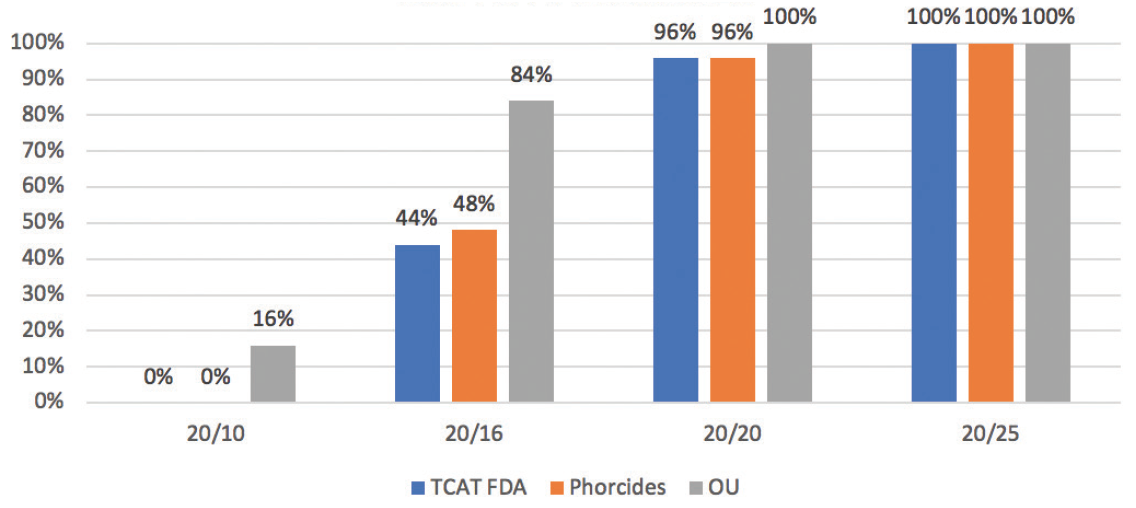
Figure 3. Postoperative day 1 outcomes in a contralateral eye study.
Courtesy of Karl G. Stonecipher, MD
Having patients see well qualitatively and quantitatively on the first postoperative day is essential. In my experience, patients expect excellent vision the day after surgery. How do surgeons keep raising the bar? They must continue to track postoperative outcomes any way they can. As Drs. Alpins and Majmudar point out, however, mean ORA is not to be overlooked, and, as we begin to understand dynamic accommodative astigmatism, we will continue to improve already excellent results.
Professor AlPins Replies
One point of agreement here is that a large discrepancy between corneal astigmatism and refractive cylinder makes treatment more challenging and increases the risk of a suboptimal visual outcome. A second point of agreement is that more accurate corneal measurements allow more precise treatment of corneal astigmatism and refractive cylinder through use of the corneal topographic astigmatism or CorT parameter and thus result in less ORA. In addition, reducing corneal irregularity produces a more defined corneal reading; ORA increases with increasing irregularity.9 A future installment of this series will focus on treating irregular astigmatism by reducing corneal irregularity.10
It remains to be seen how accurately Phorcides measures corneal astigmatism as a parameter for treatment, particularly because the software disregards cortical perception astigmatism. Until now in the peer-reviewed literature, CorT Total (which includes measurement of the posterior cornea) has been the most accurate method; it has consistently been closest to manifest refractive cylinder at the corneal plane, as quantified by mean ORA.11
The problem with treatments using TMR is that they do not address ORA. In recent research by Wallerstein and colleagues,12 outcomes were significantly inferior in the TMR group, so much so that the study was prematurely terminated to avoid further adverse visual outcomes.
Just as refractive and corneal values differ preoperatively, so do their parallel analyses differ postoperatively.13 There is value in examining both and observing both trends, particularly for nomogram refinement. Corneal analysis is objective, whereas refractive analysis is subjective. With a known zero target, there is thus risk of potential bias. None of the results offered by Drs. Majmudar and Stonecipher reports the remaining amount of corneal astigmatism. Regarding terminology,9 vectors and astigmatism have different properties: Vectors can only be calculated, but astigmatism can be measured. “Astigmatism vector” is therefore a confusing, unconventional term.
As Dr. Majmudar commented, the calculation of how much corneal astigmatism should be left is a crucial question, but caution should be exercised when “the preoperative evaluation does not make sense” because high ORA is likely. In these cases, the manifest refraction would likely be a less favorable option because it could leave excessive corneal astigmatism, such as in the example given of 1.55 D. Excessive corneal astigmatism could unexpectedly leave the patient with glare, ghosting, starburst, and halos, often termed GASH. The combination of one or more of these GASH symptoms plus high preoperative ORA and excessive postoperative corneal astigmatism (> 1.00 D) is aptly referred to as predictable avoidable LASIK surprise syndrome, a prevalent but underrecognized condition in the postsurgical population. Any treatment that incorporates both topographic and refractive parameters, such as vector planning using the Assort software (Assort Surgical Management Systems, a vector analysis program developed by Professor Alpins), will reduce the incidence of this syndrome by decreasing postoperative corneal astigmatism without increasing refractive cylinder.14
1. Kanellopoulos AJ. Topography-modified refraction (TMR): adjustment of treated cylinder amount and axis to the topography versus standard clinical refraction in myopic topography-guided LASIK. Clin Ophthalmol. 2016;10:2213-2221.
2. Koch DD, Ali SF, Weikert MP, et al. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38(12):2080-2087.
3. Mohammadpour M, Heidari Z, Mohammad-Rabei H, et al. Correlation of higher order aberrations and components of astigmatism in myopic refractive surgery candidates. J Curr Ophthalmol. 2016;28(3):112-116.
4. Zhou W, Stojanovic A, Utheim TP. Assessment of refractive astigmatism and simulated therapeutic refractive surgery strategies in coma-like-aberrations-dominant corneal optics. Eye Vis (Lond). 2016;3:13.
5. Ventura BV, Wang L, Ali SF, et al. Comparison of corneal power, astigmatism, and wavefront aberration measurements obtained by a point-source color light-emitting diode-based topographer, a Placido-disk topographer, and a combined Placido and dual Scheimpflug device. J Cataract Refract Surg. 2015;41(8):1658-1671.
6. Liu T, Thibos LN. Variation of axial and oblique astigmatism with accommodation across the visual field. J Vis. 2017;7(3):24.
7. Stonecipher KG, Kezirian GM. Wavefront-optimized versus wavefront-guided LASIK for myopic astigmatism with the Allegretto Wave: three-month results of a prospective FDA trial. J Refract Surg. 2008;24(4):S424-S430.
8. Stonecipher K, Parrish J, Stonecipher M. Comparing wavefront-optimized, wavefront-guided and topography-guided laser vision correction: clinical outcomes using an objective decision tree. Curr Opin Ophthalmol. 2018;29(4):277-285.
9. Alpins N. Practical Astigmatism: Planning and Analysis. Thorofare, NJ: Slack; 2017.
10. Alpins N, Ong JKY, Stamatelatos G. Role of hemidivisional corneal topographic astigmatisms (CorTs) in the regularization and reduction of irregular astigmatism. Cornea. 2018;37(3):386-393.
11. Alpins N, Ong JKY, Stamatelatos G. Corneal topographic astigmatism (CorT) to quantify total corneal astigmatism. J Refract Surg. 2015;31(3):182-186.
12. Wallerstein A, Gauvin M, Qi SR, et al. Primary topography-guided LASIK: treating manifest refractive astigmatism versus topography-measured anterior corneal astigmatism. J Refract Surg. 2019;35(1):15-23.
13. Alpins N. Astigmatism analysis by the Alpins method. J Cataract Refract Surg. 2001;27(1):31-49.
14. Arbelaez MC, Alpins N, Verma S, et al. Clinical outcomes of laser in situ keratomileusis with an aberration-neutral profile centered on the corneal vertex comparing vector planning with manifest refraction planning for the treatment of myopic astigmatism. J Cataract Refract Surg. 2017;43(12):1504-1514.