It is desirable to maintain a stable anterior chamber depth while performing phacoemulsification in order to minimize complications such as damage to the cornea, iris, or posterior capsule. A principal factor in maintaining a stable anterior chamber depth is controlling the IOP during phacoemulsification so that it stays within or close to the physiologic range.1 This article reviews some strategies for maintaining a stable anterior segment during cataract surgery.
MATCHING INFLOW TO OUTFLOW
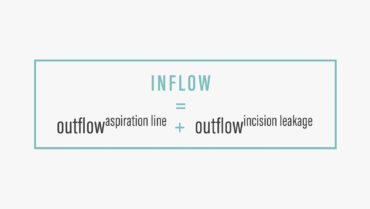
Significant fluctuations in IOP can occur during cataract surgery,2 due in part to changes in aspiration and infusion flow rates. To maintain a relatively stable anterior chamber pressure during phacoemulsification, the inflow of balanced saline solution through the irrigation line must match the outflow of fluid from the aspiration line plus the leakage from the incisions. This can be expressed mathematically as shown in the Figure.
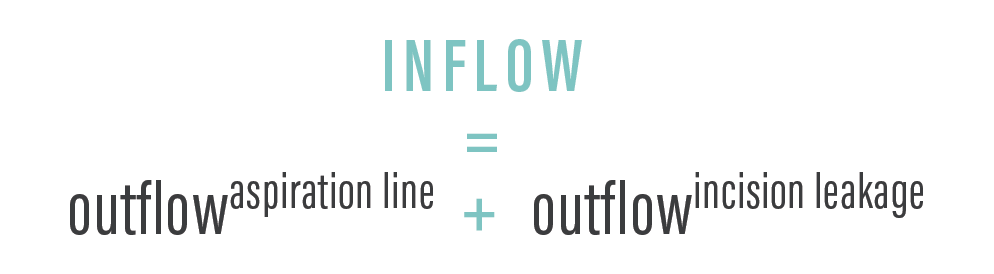
Figure. To maintain stable anterior chamber pressure during phacoemulsification, the inflow of balanced saline solution through the irrigation line must match the outflow of fluid from the aspiration line plus leakage from the incisions.
Until recently, irrigation line inflow was controllable mainly by raising or lowering the irrigation bottle because most phaco systems have had gravity-based infusion. With gravity-based systems, the primary limitation is that IOP varies inversely with the aspiration flow rate: Increasing the aspiration flow rate decreases IOP, and decreasing the aspiration flow rate increases IOP.3
With the Centurion Vision System (Alcon), the surgeon now has the option of using traditional gravity-based infusion or the active infusion system (Active Fluidics). For active infusion, motor-controlled plates squeeze and release a compliant irrigation bag to compensate for changes in flow rate and pressure as monitored by in-line sensors.3 The Active Fluidics feature actively monitors and maintains the intraoperative pressure. It has been shown in the laboratory setting to outperform gravity-based infusion systems, maintaining target IOPs across a range of aspiration flow rates.3 Other companies are following suit by developing active infusion capabilities on their phaco machines.
CONTROLLING SURGE
Another primary factor in maintaining a stable IOP during phacoemulsification is controlling postocclusion surge. Surge ensues when fluid rushes into the aspiration port of the phaco needle after the phaco tip has cleared a nuclear fragment, causing a transient IOP drop and shallowing of the anterior chamber and bringing the posterior capsule anteriorly and closer to the phaco tip.
Postocclusion surge has been partially mitigated traditionally by microcomputer systems that slow the speed of the peristaltic or venturi pump as vacuum builds while a nuclear fragment is embedded on the phaco tip. With active infusion, irrigation can be instantaneously increased to prevent surge by keeping the IOP stable. Accordingly, active infusion technology has the potential to make phacoemulsification safer compared to gravity-based systems.
OTHER APPROACHES
For those who use gravity-based phaco systems, other ways to help maintain stable IOP include decreasing the size of the phaco needle to 21 gauge, which helps to restrict fluid outflow from the eye, and switching to less compliant tubing. Changing from a 20-gauge to a 21-gauge tip has the negative consequence of slowing the removal of nuclear material, thereby decreasing efficiency but improving safety.
Also, phaco machine settings can be optimized to help minimize surge by slowing the pump speed as vacuum builds when a nuclear fragment is on the phaco tip.
INCISION SIZE
To help control outflow through the incision, it is necessary to have an incision size that corresponds with the size of the phaco needle and sleeve. I prefer to use a 20-gauge phaco needle and sleeve, and therefore I favor a 2.5-mm incision. If one uses a 21-gauge needle and corresponding sleeve, then an incision of 2.2 to 2.4 mm would be better for limiting unwanted incision fluid leakage and iris prolapse.
I also prefer diamond blades over metal blades for wound construction due to the ease of creation and the reproducibility of achieving wounds 2.5 mm by 2 mm in size. The size of paracentesis incisions should also be minimally larger than one’s desired sideport instrument and usually no bigger than 1 mm.
Femtosecond laser incisions are very precise, but frequently they cannot be placed as peripherally on the cornea as I prefer. These laser incisions can also be difficult to open initially.
CONCERNS
There are some concerns about maintaining too high an IOP with active infusion, including the possibility of ocular discomfort, postoperative corneal edema, decreased ocular perfusion, and accelerated glaucomatous optic nerve damage.4,5 In addition, using a different active infusion platform, I experienced a case in which too much inflow caused significant deepening of the anterior chamber and zonular stress in a patient with missing zonules. This necessitated a switch to less aggressive gravity-based infusion during the case. Therefore, the need for gravity-based infusion may persist for eyes that have compromised zonules.
In other instances, a patient’s anterior chamber may shallow intraoperatively, as in the event of posterior fluid misdirection syndrome, suprachoroidal effusion, or suprachoroidal hemorrhage.6 If the reason for a shallow anterior chamber is unknown, basic steps should be followed, such as increasing the infusion pressure, making sure the incisions are not excessively leaky, and verifying that there is no pressure on the globe from an expanded lid speculum. If the globe is firm despite these measures, the surgeon should look at the red reflex to evaluate for the possibility of suprachoroidal hemorrhage or effusion.6 If either is suspected, then indirect ophthalmoscopy should be performed for confirmation.6
If no evidence is seen, then a vitreous tap with a 20- to 23-gauge needle through the pars plana can be performed under direct visualization to remove some liquid vitreous.6 As an alternative, a vitrectomy tip may be inserted into the vitreous through the pars plana to cut and aspirate some vitreous while the anterior chamber is simultaneously reformed with balanced saline solution or an OVD.6
If the eye regains firmness or the anterior chamber shallows, preventing continuation of the case, prudence dictates closing the incisions with sutures and waiting hours or days for resolution before completing the case.
CONCLUSION
Diligently controlling IOP to keep it within or close to the physiologic range during phacoemulsification usually enables cataract surgery to be performed safely despite a shallow anterior chamber. Active Fluidics seems to offer a step forward in helping to maintain a stable anterior chamber during phacoemulsification. Additional clinical studies are needed to confirm the validity and safety of this new modality.
1. Seibel BS. Phacodynamics: Mastering the Tools and Techniques of Phacoemulsification Surgery. 4th ed. Thorofare, NJ: Slack; 2005:12.
2. Zhao Y, Li X, Tao A, et al. Intraocular pressure and calculated diastolic ocular perfusion pressure during three simulated steps of phacoemulsification in vivo. Invest Ophthalmol Vis Sci. 2009;50;2927-2931.
3. Nicoli CM, Dimalanta R, Miller KM, Experimental anterior chamber maintenance in active versus passive phacoemulsification fluidics systems. J Cataract Refract Surg. 2016;42(1):157-162.
4. Findl O, Strenn K, Wolzt M, et al. Effects of changes in intraocular pressure on human ocular haemodynamics. Curr Eye Res. 1997;16:1024-1029.
5. Vasavada V, Raj SM, Praveen MR, et al. Real-time dynamic intraocular pressure fluctuations during microcoaxial phacoemulsification using different aspiration flow rates and their impact on early postoperative outcomes: a randomized clinical trial. J Refract Surg. 2014;30:534-540.
6. Jick SL, Beardsley TL, Brasington CR, et al. Complications of Cataract Surgery. Book 11; Chapter 8. Basic and Clinical Science Course. San Francisco: American Academy of Ophthalmology; 2017-2018: 134-135.