In November 2017, Verana Health (formerly DigiSight Technologies) joined industry-leading investors and the leadership of the AAO to usher in a new era of big data health care analytics, using the world’s largest specialty clinical data registry—the Intelligent Research in Sight (IRIS) registry. This 6-year longitudinal data set, updated every 2 weeks, aggregates de-identified patient data from around 232 million patient encounters (53 million unique patients) integrated from more than 55 electronic health record (EHR) systems covering 14,945 Academy ophthalmologists and affiliated optometrists.1
AT A GLANCE
- The AAO and Verana Health are partnering to provide state-of-the-art data analytic tools for member doctors to help them with their daily practices.
- High-quality data are important for generating useful insights from the IRIS registry.
The goal, spearheaded by Academy CEO David W. Parke II, MD, along with multiple taskforce members and the Board of Trustees, was to develop a sustainable way to blend commercial data analytic efforts while providing value tools to Academy members at no cost. Maintaining and continuously updating the IRIS registry had become too expensive, so the Academy sought a licensing arrangement with a commercial partner.
Currently, aggregated medical data are locked in multiple data silos, either at the single EHR level (eg, EPIC Systems) or the health system level (eg, Kaiser Permanente), leaving most researchers to explore separate, smaller, prospectively enrolled, independent clinical registries, Medicare/US Department of Veterans Affairs databases or claims/prescription data from payers. Verana’s analytics, applied to clinical data from the IRIS registry, provide unprecedented nationwide information on eye care delivery.
Whenever health care data are shared, there is concern about potential HIPAA violations. For that reason, the data repository from the more than 55 EHR systems is specially designed to assign unique identifiers that track individual patients within the IRIS Registry as they move among different physicians without exposing any protected health information. Likewise, any physician identifiers are also masked based on the premise that only aggregate data—not individual data—are shared. Finally, registry data may only be used for scientific purposes and in a manner consistent with the Academy’s mission to patients and its membership, which is why there is continued oversight by the Academy’s Board of Trustees.
BENEFITS
Why should individual physicians share data? The primary drivers behind a registry run by an ophthalmic society are clinical benchmarking and quality improvement. How can eye care be improved if data among all practitioners are not collected and measured?
Additionally, the US medical reimbursement system is transitioning to quality reporting under the Merit-Based Incentive Payment System (MIPS). Academy leadership had the foresight to establish its own MD-directed, consensus-based, physician quality reporting metrics to help its members rather than wait for nonphysician regulators to define the metrics. IRIS registry participants voluntarily submit their data for MIPS reporting in order to avoid penalties and receive incentives.
Although judging quality in patient care is challenging and could include hidden biases, a countrywide physician and patient longitudinal data set is an incredible opportunity. For example, when new medications or surgical procedures are introduced, physicians initially have only FDA clinical trial data regarding safety and efficacy. The IRIS registry can aggregate peer data on treatments. Granted, the data are not as error-free as those from a randomized controlled trial, but they can still provide insight into comparative treatment outcomes, postmarket approval surveillance, gaps in preferred practice patterns, and a natural history of disease in the real world of poor compliance, economic strictures, variable disease progression, etc.
For descriptive statistics of various conditions and treatments, the IRIS registry is an invaluable resource. Recently, the FDA was able to use IRIS registry data to assess the relative safety profiles of different anti-VEGF therapies. Data analysis revealed no statistically significant differences in rates of endophthalmitis among these therapies. Retrospective database mining research can spur additional prospective research such as studies of which types of patients respond best to which anti-VEGF agent.
MESSY REAL-WORLD DATA
Physicians who work with EHR software know that recorded data are not perfect. There are typos, copy-and-paste mistakes, too much subjective grading, non–evidence-based habits, omissions, biased incentives, inaccuracies entered only for coding and billing requirements, etc. These data may not reflect the ground truth for various diagnostics and therapeutics. For this reason, Verana Health assembled a professional team of domain experts in clinical ophthalmology, medical affairs, practice management, reimbursement, coding, marketing, clinical trials, and data science.
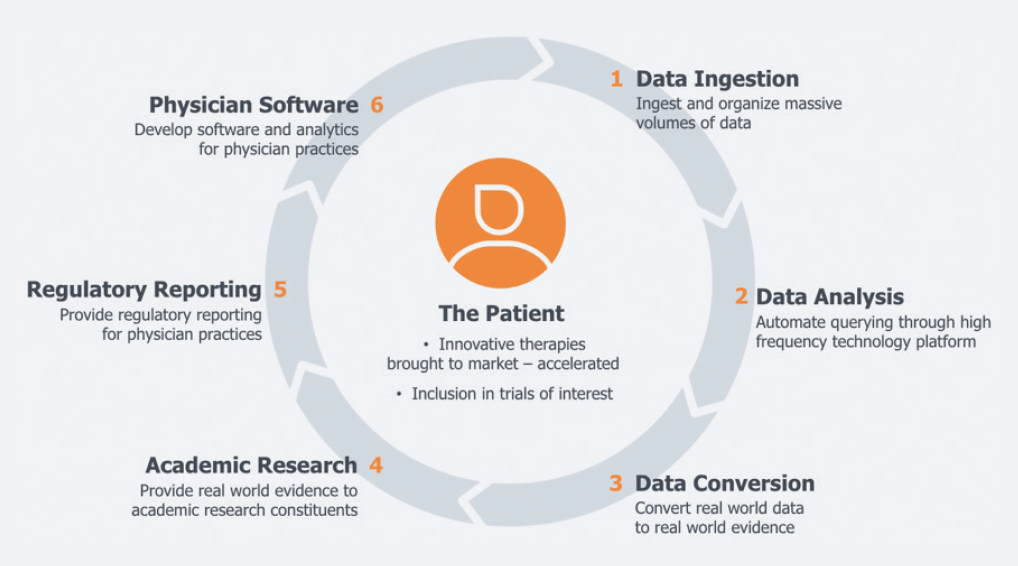
Figure. Through the Verana Health partnership, participation in the IRIS registry returns meaningful and sustainable value to physicians and patients alike.
Obtaining useful answers from data requires asking the right questions. In order to organize and create a clean data set across EHR types, Verana Health has established a standardized process to enhance the data to a standard at which accurate analysis can be applied to an optimized database. This involves a curation process based on the core constructs of checking for accuracy, completeness, consistency, credibility, and relevance of data across technical, clinical, and scientific dimensions.
One of the benefits of a very large data set is that confirmatory triangulation data points can be used to verify the accuracy of the data. For example, if a de-identified patient had cataract surgery coded by Current Procedural Terminology code, a data scientist could confirm eye laterality by cross-referencing the structured fields in the preoperative and postoperative notes in addition to the operative note text. Prior Medicare database studies evaluating a procedure with a specific Current Procedural Terminology code to identify any associated complications could not achieve this level of granular detail. Of course, the IRIS Registry is not designed to prove causality for any discovered associations, but the opportunity to incorporate results from other colleagues (wisdom of the crowd) can help improve outcomes.
VALUE TOOLS
In addition to focusing on benefits for research and commercial uses, the Verana clinical team is creating value tools for Academy members who contribute their EHR data to the IRIS registry. Starting with the most common eye conditions, Verana, with the help of physician feedback, is crafting analytic dashboards that include treatment pathway visualizations, longitudinal real-world outcomes, prescription persistence trends, and aggregate cost-effectiveness analyses. Doctors can then compare their own results against those of the entire IRIS registry cohort. Verana is also developing a clinical trial dashboard that will help practice managers and principal investigators identify candidate study patients within their practice populations.
More information will be available at a course entitled “Member Value Tools From the IRIS Registry and Verana Health: How to Improve Your Practice” during the AAO Annual Meeting in San Francisco this fall.
1. IRIS Registry Statistics. January 1, 2019. FIGmd.