
Keratoconus (KCN) is a progressive corneal disorder characterized by thinning and distortion of corneal curvature, which results in refractive change and induces high and often irregular astigmatism. In general, KCN manifests during early puberty, and in a small percentage of patients, it may eventually necessitate penetrating keratoplasty. CXL involves the saturation of the corneal stroma with riboflavin and irradiation with UV-A light, which strengthens and frequently flattens the cornea through the formation of chemical bonds.1 Since CXL became available, the number of keratoplasties performed for KCN has declined significantly, which is likely a positive downstream effect of earlier treatment and stabilization.2 When the patient is pregnant, managing KCN and offering CXL become more complex.
AT A GLANCE
- Use of CXL to treat keratoconus during pregnancy involves assessing the risk-benefit ratio when the benefit is well understood but the risk is not.
- Current evidence will not help clinicians decide how to proceed with pregnant or lactating patients. It is not only CXL itself but also the necessary medications to be used in conjunction with the procedure that must be considered.
CASE EXAMPLE
A 32-year-old woman is referred by her optometrist for evaluation of a corneal irregularity detected during her most recent annual examination. The patient has no ocular history and reports that she has been wearing spectacles for the past 6 years with no change in prescription. She reports, however, that she has recently noticed a progressive worsening of vision in both eyes. Refraction shows a significant myopic and astigmatic shift. Testing with the Pentacam (Oculus Optikgeräte) gives maximum keratometry readings of 60.30 D OD and 55.40 D OS (Figure).
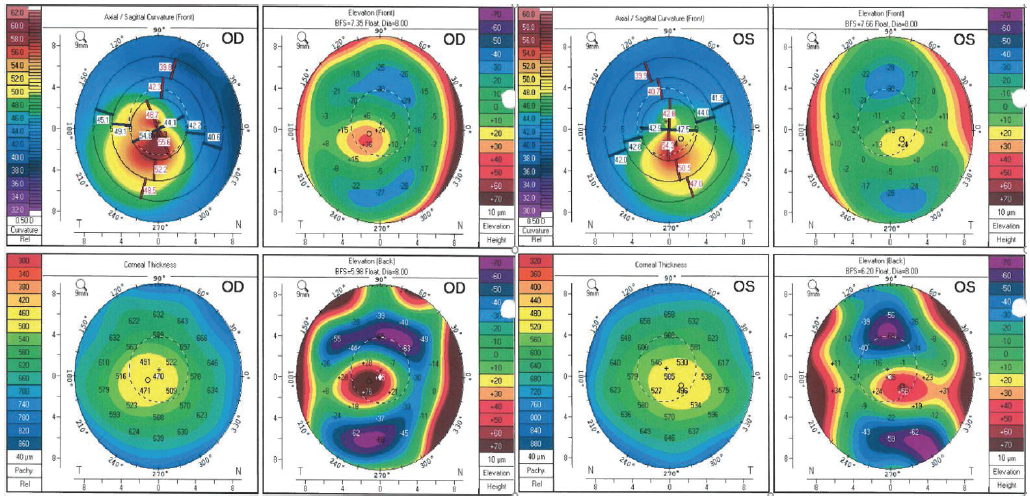
Figure. Pentacam images of a 32-year-old woman with previously undiagnosed KCN and recent vision changes in each eye. No prior images with this device are available. At the time that these images were acquired, the patient was 20 weeks pregnant.
It is explained to the patient that the examination findings and imaging are consistent with KCN, and options are described for treatment, including CXL. After discussing management in full detail and scheduling the patient for surgery, she asks if the procedure will harm her baby. She is 20 weeks pregnant.
Having listened to a description of the importance of CXL for stabilizing progressive KCN, the patient is understandably anxious about undergoing the procedure and worried about her long-term visual prognosis. How should you proceed?
THE KNOWNS AND THE UNKNOWNS
Use of CXL to treat KCN during pregnancy involves assessing the risk-benefit ratio when the benefit is well understood but the risk is not. Pregnant and lactating patients were excluded from all FDA clinical trials in the approval process of CXL, and no published studies or reports currently address the matter. Not only is the safety of the procedure in this patient population unknown, but so is its effectiveness.
Pregnancy induces a variety of hormonal changes that affect tissues throughout the body, and the eyes are no exception. The physiologic ocular changes of pregnancy can induce a myopic shift, and they may relate to steepening of the corneal curvature.3 A statistically significant steepening of previously normal corneas has been observed in the second and third trimesters and has been found to resolve after delivery or cessation of nursing.4 The change is thought to result from the rise in pregnancy-related hormones, namely estrogen and relaxin, which may alter the biomechanical properties of the cornea and increase its refractive power.5,6
In the setting of KCN, it can be difficult to discern true pregnancy-related progression from these transitory changes of the cornea. Investigators in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study reported that, in their patient cohort, they could not determine a hormonal role in KCN progression, although they drew this conclusion by studying women aged 48 to 59 on hormone replacement therapy.7 Several other reports, however, have suggested a connection between pregnancy and KCN progression. One of the first associations between ectasia and pregnancy was described in a patient with a history of LASIK; she experienced dramatic corneal steepening only after her first pregnancy, and the ectasia progressed again after her second pregnancy despite interim CXL.8 In another report of seven eyes of four pregnant patients with previously stable KCN, Bilgihan and colleagues reported progression in myopic refraction and simulated keratometry metrics starting in the third trimester, which also happens to be the gestational time at which estrogen levels are known to be the highest.9 Although follow-up was not complete for all of the women, one of the patient’s conditions was found to stabilize spontaneously after delivery, and another was subsequently treated with CXL. It is unclear if pregnancy-related changes in KCN eyes are transient, but reports have suggested that topographic progression in pregnancy may be reversible, similar to that described in healthy eyes.10
The most illustrative study on the matter is a case-controlled report on 22 patients with bilateral KCN, 11 of whom intended to become pregnant and 11 of whom did not.11 The study compared refractive and topographic data once before pregnancy, once during the third trimester, and once 6 months postpartum. The researchers found that 100% of eyes in the pregnancy group experienced significant KCN progression, whereas none of the control eyes demonstrated disease progression. In this report, no evidence of reversal after pregnancy was found, and no long-term follow-up outcome was presented.
To sum up, current evidence will not help you decide how to proceed in the case presented earlier. The FDA label for the Photrexa Viscous/Photrexa/KXL System states, “It is not known whether the corneal collagen cross-linking procedure can cause fetal harm or affect reproduction capacity” and recommends against performing CXL on pregnant patients.12 Similarly, it is not known if the drug or procedure has negative effects on breastfeeding infants, and the use of clinical judgment is recommended.
It is not only the procedure itself but also the necessary medications to be used in conjunction with the procedure—namely topical antibiotics and steroids—that must be considered. For example, I routinely prescribe a topical fluoroquinolone and prednisolone with or without a nonsteroidal antiinflammatory drug after CXL, all of which are category C drugs and have demonstrated adverse fetal effects in animal studies.13 Despite their topical application, I assume some level of systemic absorption and prefer to follow FDA recommendations by avoiding use of these drugs during pregnancy if possible.
HOW TO PROCEED
What, then, is the recommendation for the patient in this case? To recap, she is young and approaching the third trimester of her pregnancy. She is previously undiagnosed with KCN, but imaging strongly suggests the disease now. Some evidence exists for a gestational role in KCN progression, and this patient might have had subclinical KCN before pregnancy. It is unknown, however, if her disease will stabilize after delivery or continue to progress. Because of this unknown and the unknown risk to her child of performing the procedure, I believe that CXL is not advisable for her. Instead, I would recommend close monitoring, and I would spend extra time explaining the importance of avoiding eye rubbing, even if she denies rubbing her eyes. I might even consider recommending that she wear nocturnal protective goggles to avoid unintentional eye rubbing or pressure from pillow contact because side sleeping is an eventuality for these patients.
In terms of refractive management, I would try to maintain the patient in spectacles as long as she was functional. Contact lenses could also be considered. In addition, I would explain that her refractive error may change after delivery and lactation. Finally, I would likely monitor her closely with refraction and topography some time in the third trimester and again after delivery, and I would suggest she undergo CXL before considering a future pregnancy.
CONCLUSION
Challenging scenarios call on clinicians to understand the available data and to guide pregnant or lactating patients in a way that minimizes the risk to both them and their babies. Female patients with documented or suspected KCN should be asked about their intentions to become pregnant and considered for CXL in a prophylactic manner.
1. Galvis V, Tello A, Ortiz AI, Escaf LC. Patient selection for corneal collagen cross-linking: an updated review. Clin Ophthalmol. 2017;11:657-668.
2. Sandvik GF, Thorsrud A, Råen M, et al. Does corneal collagen cross-linking reduce the need for keratoplasties in patients with keratoconus? Cornea. 2015;34(9):991-995.
3. Balasubramanian K, Mathiyalagan S, Nagarajan G. A prospective study of changes in the refractive system of eye during pregnancy. Int J Sci Study. 2017;5(4):89-92.
4. Park SB, Lindahl KJ, Temnycky GO, Aquavella JV. The effect of pregnancy on corneal curvature. CLAO J. 1992;18(4):256-259.
5. Suzuki T, Kinoshita Y, Tachibana M, et al. Expression of sex steroid hormone receptors in human cornea. Curr Eye Res. 2001;22(1):28-33.
6. Spoerl E, Zubaty V, Raiskup-Wolf F, Pillunat LE. Oestrogen-induced changes in biomechanics in the cornea as a possible reason for keratectasia. Br J Ophthalmol. 2007;91(11):1547-1550.
7. Fink BA, Sinnott LT, Wagner H, et al; CLEK Study Group. The influence of gender and hormone status on the severity and progression of keratoconus. Cornea. 2010;29(1):65-72.
8. Hafezi F, Iseli HP. Pregnancy-related exacerbation of iatrogenic keratectasia despite corneal collagen crosslinking. J Cataract Refract Surg. 2008;34(7):1219-1221.
9. Bilgihan K, Hondur A, Sul S, Ozturk S. Pregnancy-induced progression of keratoconus. Cornea. 2011;30(9):991-994.
10. Hoogewoud F, Gatzioufas Z, Hafezi F. Transitory topographical variations in keratoconus during pregnancy. J Refract Surg. 2013;29(2):144-146.
11. Naderan M, Jahanrad A. Topographic, tomographic, and biomechanical corneal changes during pregnancy in patients with keratoconus: a cohort study. Acta Ophthalmol. 2017;95(4):e291-e296.
12. Photrexa and Photrexa Viscous [package insert]. Waltham, MA: Avedro; 2016.
13. FDA pregnancy categories. DHHS Website. September 29, 2017. https://chemm.nlm.nih.gov/pregnancycategories.htm. Updated September 29, 2017. Accessed December 10, 2018.




