Nonadherence to treatment regimens is endemic in glaucoma. Up to 80% of patients with glaucoma may not use topical medication as prescribed.1 Poor adherence is associated with worsening vision and higher overall health care costs.2 Because barriers to adherence include patient forgetfulness, difficulty administering eye drops, and the burden of dosing frequency,1 there is an unmet need for glaucoma therapy that does not require daily eye drop instillation.
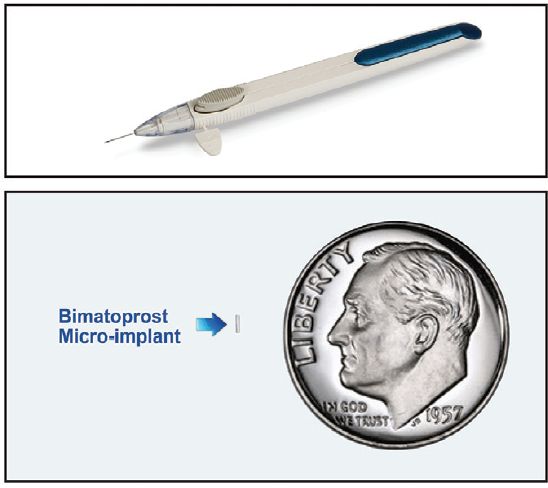
Figure. Bimatoprost SR single-use applicator (top). Implant positioned next to a dime for size comparison (bottom).
STUDY RESULTS
1. Olthoff CM, Schouten JS, Van de Bourne BW, et al. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112:953-961.
2. Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44.
3. Lewis RA, Christie WC, Day DG, et al. Bimatoprost sustained-release implants for glaucoma therapy: 6-month results from a phase I/II clinical trial. Am J Ophthalmol. 2017;175:137-147.
4. Craven R, Walters T, Christie WC, et al. 24-month phase 1/2 clinical trial of bimatoprost sustained-release implant (bimatoprost SR) in glaucoma patients. Paper presented at: American Academy of Ophthalmology Annual Meeting; November 11-14, 2017; New Orleans.
5. Allergan announces positive topline phase 3 clinical data for Bimatoprost SR (sustained-release) implant for IOP lowering in patients with open-angle glaucoma or ocular hypertension. https://www.allergan.com/news/news/thomson-reuters/allergan-announces-positive-topline-phase-3-clinic.aspx. Accessed May 16, 2019.
6. Allergan announces positive 3-month topline results from second phase 3 study of Bimatoprost SR (sustained-release) implant for lowering intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. https://www.allergan.com/news/news/thomson-reuters/allergan-announces-positive-3-month-topline-result. Accessed May 16, 2019.




