
Identifying a need and putting the puzzle pieces together to satisfy the associated market demand are perhaps the world’s second oldest profession, and it is one that serial ophthalmic entrepreneur and physicist David Muller, PhD, is—for lack of a better term—winning. Dr. Muller first lit the ophthalmic world on fire with the Summit laser for PRK and more recently made corneal collagen cross-linking an exciting option in the refractive realm with PiXL (Avedro), which expanded cross-linking beyond its limited keratoconus indication (recently approved by the FDA, see EyewireTV for more: bit.ly/1SUhh5R). Now, he is poised to disrupt the corneal inlay space with tissue addition technology aimed specifically, but not solely, at satisfying the refractive demands of the enormous and growing presbyopia market.

Dr. Muller’s newest offering is TransForm allogenic tissue inlays and onlays. The product is in development by Allotex, a startup company helmed by Dr. Muller in conjunction with well-known ophthalmic innovator Michael Mrochen, PhD. “Allotex is taking pieces from the past, sewing them together, and making a commercial offering of what already exists,” says Dr. Muller.
THE BACKGROUND
Dr. Muller’s latest “a-ha” offering combines vintage theories and contemporary capabilities. The concept of using tissue addition for refractive purposes was actually the very first form of refractive surgery, dating back to José Barraquer, MD, who made his first attempt in 1949. “The basic idea was to take donor tissue, reshape it, and place it on the eye,” notes Dr. Muller. “The first attempts were very crude and unsuccessful, but from as early as the mid-1980s, it has been shown that human donor corneal tissue can be safely added to a native cornea.”
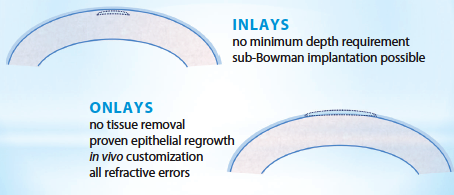
Figure 1. Benefits of allogenic inlays and onlays.
The problem was that there were no tools precise enough to achieve a predictable refractive change. “Barraquer along with other early pioneers, including Swinger and Krumiech, worked on systems to better shape donor tissue,” Dr. Muller recalls. “First, there was a cryolathe that froze the tissue in preparation for sculpting, and later, there were mechanical keratomes that were used to sculpt the tissue without freezing. There was even an attempt by Allergan to develop a commercial freeze-dried product called the ‘Kerato-Lens’ that could be rehydrated and shaped. None of these techniques worked out because the clinicians were unable to shape the tissue accurately enough to get a predictable refractive change.” Because of this limitation, the development of tissue addition technology swung to the use of synthetics.
Since that time, developments have effectively ended the reliance on synthetics. “First, it’s now possible to cut corneal tissue very accurately, given the availability of excimer and femtosecond lasers and surgeons’ expertise in using this equipment,” Dr. Muller says. “Second, the recent development in eye banking that enables human corneal tissue to be safely kept in a package at room temperature, for up to 2 years, permanently changes the paradigm.”
The TransForm inlays are optically comparable to synthetic corneal inlays, but because allogenic implants are natural corneal tissue, they avoid the biocompatibility issue often associated with synthetic corneal inlays, Dr. Muller states. “Advances in laser technology and corneal tissue management have now reached the point where we can offer allograft implants, made from human donor corneas, in an ethical and affordable fashion,” he says.
THE PLAN
TransForm is in its early stages of development, but Dr. Muller expects the product to be on the market, on a limited basis, within about 12 to 18 months in the United States and abroad. The plan is for Allotex to acquire eye-banked corneal tissue and then process it into extremely thin sections so that each cornea can ultimately become multiple inlays (Figures 1-3). From there, the tissue will be provided to surgeons who use their excimer or femtosecond laser—or a “desktop” excimer laser currently in development by Allotex—to alter the shape to provide the appropriate refractive correction for the patient. Unlike artificial inlays, there is no minimum depth requirement for Transform inlays, and sub-Bowman placement is possible. For onlays, no tissue removal is necessary, and epithelial regrowth has been previously demonstrated to work, according to Dr. Muller.

Figure 2. A demonstration of the femtosecond laser’s ability to make thin sheets from a cornea (early development: porcine laminar sheets).
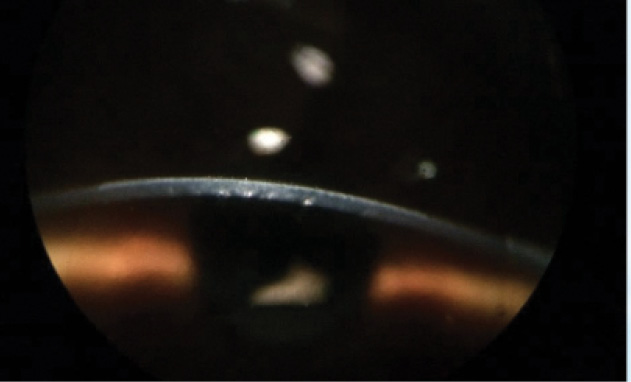
Figure 3. First clinical result: myopic onlay treatment (immediately after surgery with -5.00 D correction and a 6-mm optical zone).
It is commonly thought that there is a shortage of corneal tissue, when in fact the shortage is really a paucity of healthy endothelial tissue, Dr. Muller says. “Because we can make many corneal sections from a single cornea, there is an ample supply of corneal tissue that is not useable for transplant[ation] and would most likely go to waste if we didn’t use it,” he says. Allotex has an exclusive arrangement with the Lions VisionGift in Portland, Oregon, which produces the specially processed corneas.
Dr. Muller explains that, from a regulatory standpoint, “we can’t supply corneal lenticules that have a prescription in them, because we can’t make any claims about what refractive powers these implants will make. So rather than doing that, we’re supplying the surgeons with the corneal tissue from which they can make patient-specific corneal implants. Separately, we are also developing a specialized laser system that can be used to reshape tissue ex vivo. [Physicians] could, in fact, use excimer laser systems that they have currently to reshape the tissue, but we are developing an ex vivo system to sculpt the tissue that might make it a little bit easier for them to use.”
Corneal allografts are regulated as what is called a 361 product, which means that there are no specific clinical trials required. “We will be providing the ophthalmologist with small pieces of corneal tissue that they can subsequently shape, using several possible laser [platforms],” explains Dr. Muller. “As soon as we can start producing them, we can start selling them.”
So far, the project is self-funded. “The funds needed and time to market [are] actually quite small,” he says. “We’re actually very far up the learning curve because of the 30 years of work of reshaping the corneas with lasers as well as work that’s been done more recently with SMILE implants [a form of flapless refractive laser surgery that was adapted from refractive lenticule extraction or ReLEx from Carl Zeiss Meditec (not approved in the United States)]. The other thing we know is that TransForm allografts are incredibly safe and 100% removable without leaving any residual damage behind.”

Corneal and refractive specialists Peter Hersh, MD, and Vance Thompson, MD, are working with Allotex in an advisory capacity. Dr. Hersh says TransForm’s existence is the result of an evolution in tissue processing as well the ability of femtosecond lasers to potentially process tissue on a large scale. “Advances in eyebanking and corneal tissue preparation combined with the high technology of femtosecond lasers have really brought all of this to the fore,” he explains.
THE BENEFITS
The benefits are obvious, according to Dr. Muller. He says TransForm is guaranteed to be biocompatible, “whereas developers of synthetic inlays have to ensure that their inlays don’t deprive the anterior cornea of nutrition and oxygen.” He adds, “Those companies have approached this problem by making the inlays with very small diameters and burying them very deep inside the cornea. This allows the nutrients to flow around the inlay in a manner that prevents them from being rejected; nonetheless, they are a foreign body that the body will continue to try to reject.” TransForm inlays and onlays can be of any diameter, which he says affords several benefits.
“[The inlays] provide great flexibility on optical zone size, can be placed at any depth that is advantageous for getting the proper shape response, and if they are removed, it is no different than removing native tissue,” comments Dr. Muller. Not one to stop at the obvious, he goes on to suggest that the big story here is that these allografts have applications beyond presbyopia, in hyperopia and even myopia.
“This has a pretty large platform, potentially even for the correction of irregular corneas,” says Dr. Hersh. “Looking ahead to the future, I could even see it being done adjunctively with cross-linking for keratoconus treatment.”
THE MARKET
The presbyopia market is hungry for refractive solutions that enable patients to escape eyeglasses, do not require them to undergo surgery, and are easily reversible. Dr. Muller thinks he has nailed it with TransForm. He says being able to place an onlay under the epithelium, informing patients that the epithelium will recover within about 6 hours, and explaining that, if for any reason they are not satisfied with it, they can easily have it removed would be an attractive option.
Dr. Muller expects hyperopia to be a hot market for this option as well and anticipates that myopia, too, will fall in line. He says TransForm represents an alternative to synthetic implants for presbyopia and LASIK for hyperopia and myopia. He asserts, “It’s not going to do away with LASIK, but it is going to expand the refractive surgery market.”
THE LIGHTBULB
Dr. Muller came up with the idea to develop allografts as corneal inlays for refractive correction about 2 years ago and began putting the backbone of the company in place. The next step was to bring Dr. Mrochen in and set up shop. “Michael will be setting up a technology development group in Zurich, and I will be setting up the commercial facility in Boston,” says Dr. Muller. “We will be focused on developing the specialized laser technology and processing systems as well as getting into production, but at this stage, it can be said without a doubt that the TransForm allografts will be safe by any reasonable standard. We know that tissue can accurately be shaped, and we believe that our track record of developing similar technology gives us a very high likelihood of being successful.”
Rochelle Nataloni
• freelance medical writer
• (856) 401-8859; rnk2020@comcast.net; Twitter @JustRochelleN; www.linkedin.com/in/rochellenataloni
David Muller, PhD
• CEO of Allotex, Boston
• david@allotex.com
Peter S. Hersh, MD,
• Cornea and Laser Eye Institute, Hersh Vision Group, Teaneck, New Jersey
• (201) 692-9434; phersh@vision-institute.com
• financial interest: none acknowledged


