Case Presentation
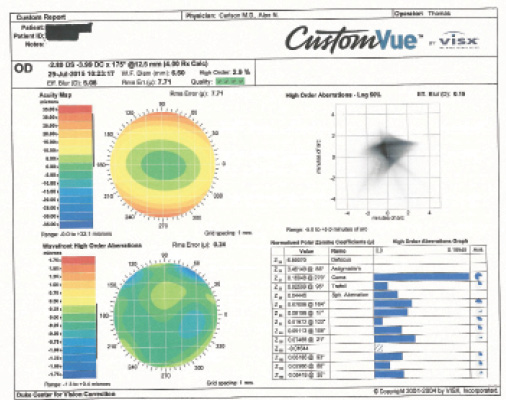
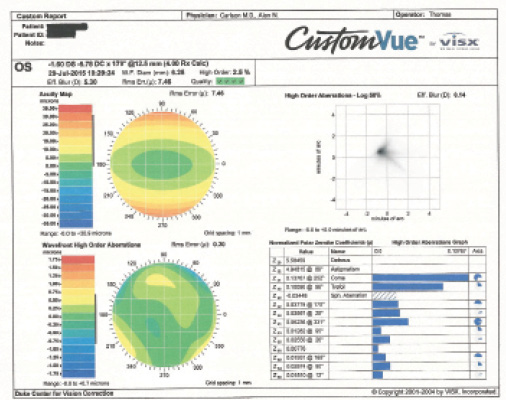
Figure 1. WaveScan measurements for the patient’s right (left) and left (right) eyes.

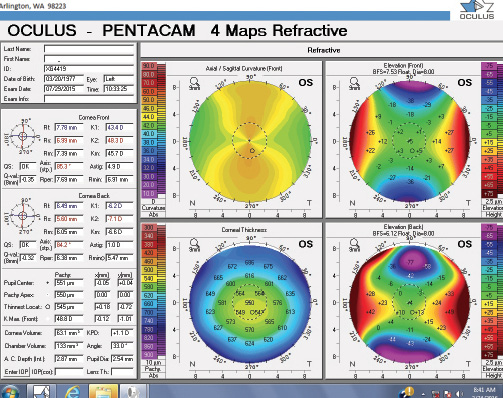
Figure 2. Measurements with the Pentacam for the patient’s right (left) and left (right) eyes.

Figure 3. Topography.
PREEYA K. GUPTA, MD

This patient’s high corneal astigmatism appears to be mostly regular, aside from some mild inferior steepening on axial topography in the left eye; there is no posterior elevation on tomography in either eye. My first step in evaluating potential refractive surgery candidates is to scrutinize the topography and tomography. Specifically, I look for the following:
- any signs of keratoconus, pellucid marginal degeneration, or forme fruste keratoconus
- more than 17 µm of posterior elevation, as measured by the Pentacam (Oculus)
- inferior steepening on topography
- any decentration of the corneal apex with associated corneal thinning
My level of suspicion also rises if the coma measures higher than 0.2 µm with the WaveScan Wavefront System (Abbott Medical Optics).
If I found no signs of corneal weakness, I would consider this patient to be a candidate for LASIK or PRK. My personal bias is towards surface ablation for anyone with suspicious findings, and this patient exhibits mild inferior steepening in her left eye along with 5.00 D of corneal astigmatism.
With surface ablation, patients are at risk of corneal haze formation. I typically use mitomycin C (MMC) during the procedure. Considering this patient’s high astigmatism, I would apply MMC to the corneal surface for 20 to 30 seconds, followed by copious irrigation with balanced salt solution to help reduce risk of corneal haze formation. Oral vitamin C supplementation can also reduce the risk of this complication, in addition to aggressive management of epithelial recovery. I tend to see these patients more frequently postoperatively to watch for the development of haze. If it starts to form early, then prolonged treatment with topical steroids is necessary. Also important is managing patients’ expectations after surface ablation for high astigmatism, because months may elapse before the cornea and refractive error stabilize, unlike after lower levels of astigmatic correction.
Patients with high regular corneal astigmatism can be excellent candidates for refractive surgery if the topography and tomography readings are normal. These individuals are often my happiest patients because of the dramatic improvement in their visual function.
GREGORY D. PARKHURST, MD

Key pieces of information in this case include:
- the patient’s age
- refractive stability
- average central corneal thickness
- magnitude of the steepest keratometry reading (< 47.00 D OD and < 48.00 D OS)
- no asymmetric corneal steepening
- predicted residual bed (319 µm OD and 326 µm OS)
The BCVA of the patient’s two eyes differs slightly, which is explained by long-standing amblyopia from asymmetric astigmatic blur.
I would offer her thin-flap LASIK using the iFS Laser (Abbott Medical Optics) and the Allegretto Wave Eye-Q excimer laser system (Alcon). The FDA approved the latter for the treatment of up to 6.00 D of myopic astigmatism—exactly double the amount of astigmatism that can be corrected using most other excimer laser systems in the United States. I have had excellent success in cases of high myopic astigmatism using this laser platform.
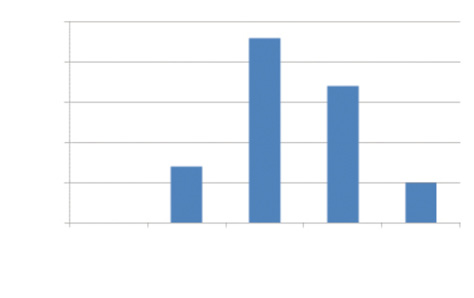
Figure 4. Safety: preoperative BCVA versus postoperative BCVA.
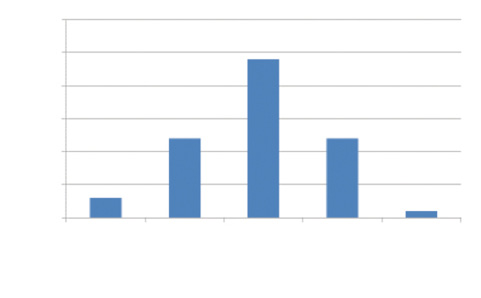
Figure 5. Efficacy: postoperative uncorrected distance visual acuity versus preoperative BCVA.
A few years ago, I queried the database (SurgiVision DataLink; SurgiVision Consultants) for high astigmatic outcomes at the military refractive surgery center where my colleagues and I performed several thousand LVC procedures annually. I specifically searched more than 10,000 outcomes for cases of high regular astigmatism, and I identified 52 treated eyes with a preoperative refraction between -4.00 and -7.50 D of myopic cylinder (Figures 4 and 5). Of those, 38 eyes (73%) were correctable to at least 20/20 preoperatively; the others were presumed to be slightly amblyopic like the left eye in this case. Among the 38 eyes that were correctable to 20/20 preoperatively, 84% achieved 20/20 distance UCVA by 1 month postoperatively, and there was a 100% rate of satisfaction in this group. My colleagues and I counsel patients like this one that they are at increased risk of having residual refractive error. Enhancement care is much better tolerated under a flap than via surface ablation.
KARL G. STONECIPHER, MD

When faced with a patient who has a high degree of ametropia, whether astigmatism or myopia, the first issue is stability. If a patient such as this one presents without previous topography and he or she is less than 35 years of age, I will wait 6 months and repeat the topography to determine stability. At 38 years of age, this patient’s astigmatism is unlikely to progress. If anything, with time, she will probably lose some with-the-rule astigmatism. Upon analysis with the Pentacam, the cylinder in the right eye is 3.70 D on the anterior surface and 0.80 D on the posterior surface; in the left eye, the astigmatism measures 4.90 D on the anterior surface and 1.00 D on the posterior surface. The manifest refraction is consistent with those numbers.
At my center, this patient would undergo preoperative testing with the Magellan Mapper (Nidek), Allegro Topolyzer Vario Diagnostic Device (Alcon), OPD-Scan III (Nidek), and iTrace (Tracey Technologies). First, the Magellan Mapper would look at 12 analytical options to evaluate the patient’s potential for ectasia using the Smolek/Klyce software for corneal diagnostics. The Allegro Topolyzer Vario Diagnostic Device provides similar evaluations, and outside the United States, most surgeons would consider a topography-guided treatment for this patient. In this country, topography-guided LVC would be an off-label option and would require two treatments. In my hands, this strategy would not be worth the risk, considering the results provided by wavefront-optimized treatments (Alcon). Both the OPD-Scan III and iTrace would allow me to differentiate among the contributions of the whole eye, the cornea, and the lens. These measurements can influence my nomogram, which I currently compile using the IBRA system (Zubisoft).
Using a wavefront-optimized treatment, my nomogram would remove between 75 and 80 µm of tissue. With a laser flap of 100 µm (standard deviation, ±4 µm), the residual stromal bed would be 350 µm. Preoperatively, I would stress to this patient that an enhancement might not be an option and that, in my hands at this level of refractive error, my enhancement rate is between 3% and 4% using the Allegretto Wave Eye-Q excimer laser system with a wavefront-optimized treatment profile. At my center, my colleagues and I have had more success in patients such as this one with LASIK as opposed to surface treatment. Our surface treatment enhancement rate for a patient with this degree of astigmatism is between 7% and 8%. That is why I would choose LASIK instead of surface treatment. If the percent tissue altered and/or the residual stromal bed were an issue, however, then I would consider surface treatment and treat her with my standard surface ablation protocol.
In patients with high regular corneal astigmatism, I treat ocular surface disease aggressively, because data presented by my group (N = 4079) showed dry eye disease to be the second most likely influence on a patient’s requiring an enhancement.1 Based on recent presentations by Randelman and Santhiago, the percent tissue altered (33%-34%), the residual stromal bed of 350 µm, and the patient’s age, the likelihood of ectasia is low.2,3 Again, however, I would stress to the patient that the option of an enhancement might not be available.
I would treat this patient with difluprednate 0.05% (Durezol; Alcon) and gatifloxacin 0.5% four times per day for 2 weeks postoperatively. If I had any concerns about ocular surface disease, I would treat it aggressively with punctal occlusion and topical cyclosporine for 1 to 2 months before surgery. I would have the patient stop the cyclosporine 3 days prior to surgery and start the difluprednate and gatifloxacin at that time. I would instruct the patient to resume cyclosporine treatment 3 days after surgery.
With patients such as this one, my colleagues and I discuss the potential for postoperative glare. We let patients know that there will be an adjustment period postoperatively, but with neuroadaptation, these are some of the happiest patients we have the opportunity to treat. At this level, 100% of our patients achieve 20/32 or better UCVA, and 31% attain 20/16 or better UCVA. Obviously, many of these patients’ preoperative BCVA is worse than 20/20, as in this case.
ALAN N. CARLSON, MD

The panelists have done a superb job of addressing a topic that recently has come under great scrutiny. A few additional generalities with regard to clinical considerations when treating patients with a high degree of astigmatism include
- What confidence does the surgeon have in the data used to confirm stability over time?
- Does the contact lens or orthokeratology history suggest leaving contact lenses out for a longer period of time?
- Is the location of the corneal apex on corneal tomography appropriately located with respect to the “line” of sight, or is there suspicious decentration?
- Is the value of corneal thickness in the normal range, and is there anticipated symmetry in the magnitude and axis of astigmatism between the patient’s two eyes?
- Are there abnormal islands of elevation on the anterior or posterior cornea by tomography?
- Is there an explanation of asymmetry of corneal astigmatism?
- Are asymmetry and progression of astigmatism associated with a history of eye rubbing or sleeping in a position that puts pressure on one or both eyes (sometimes eyelash misdirection suggests this pressure)?
- When performing LASIK, does the flap’s hinge need to be moved to manage bed size (ie, moving the hinge into the steeper axis [treating myopic astigmatism] to reduce bed size)?
- Is the astigmatism significant enough that surface ablation is more likely to cause asymmetric scarring as well as less predictable epithelial thickness and overall healing?
- Is the astigmatism predominantly in the cornea, thus avoiding a problem down the road that can occur after treating a high degree of lenticular astigmatism in the cornea?
1. Stonecipher KG, Corkin R, Influences on enhancement rates in laser vision correction. Paper presented at: XXXII Congress of the ESCRS; September 13-17, 2014; London, England.
2. Randelman JB. Evaluation of role of age, residual stromal bed, and percent tissue altered in ectasia risk assessment for patients with normal preoperative topography. Paper presented at: ASCRS/ASOA Symposium and Congress; April 17-21, 2015; San Diego, CA.
3. Santhiago MR. Role of percent tissue altered in post-LASIK ectasia with suspicious topography. Paper presented at: ASCRS/ASOA Symposium and Congress; April 17-21, 2015; San Diego, CA.
Section Editor Alan N. Carlson, MD
• professor of ophthalmology and vice chair, departmental development, Duke Eye Center, Durham, North Carolina
• (919) 684-5769; carls009@mc.duke.edu; alancarlsonmd.com
Section Editor Stephen Coleman, MD
• director of Coleman Vision, Albuquerque, New Mexico.
Section Editor Karl G. Stonecipher, MD
• clinical associate professor of ophthalmology, University of North Carolina, Chapel Hill
• director of refractive surgery, TLC in Greensboro, North Carolina
• (336) 274-2244; stonenc@aol.com
• financial disclosure: consultant to Alcon, Allergan, and Nidek
Section Editor William F. Wiley, MD
• private practice at Cleveland Eye Clinic, Cleveland, Ohio
Preeya K. Gupta, MD
• assistant professor of ophthalmology, cornea and refractive surgery, Duke Eye Center, Durham, North Carolina
• (919) 660-5071; preeya.gupta@duke.edu; Twitter @preeyakgupta
• financial disclosure: consultant to Abbott Medical Optics and Alcon
Gregory D. Parkhurst, MD
• founder and physician CEO, Parkhurst-Nuvision, San Antonio, Texas
• (210) 615-9358; gregory.parkhurst@gmail.com; Twitter @PNuVision and @gregparkhurstmd
• financial interest: none acknowledged




